Sex determination in Drosophila: The view from the top
- PMID: 20160499
- PMCID: PMC2855772
- DOI: 10.4161/fly.4.1.11277
Sex determination in Drosophila: The view from the top
Abstract
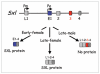
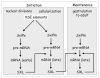
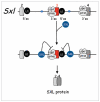
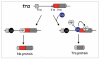
One of the most important decisions in development is whether to be male or female. In Drosophila melanogaster, most cells make this choice independent of their neighbors such that diploid cells with one X chromosome (XY) are male and those with two X chromosomes (XX) are female. X-chromosome number is relayed through regulatory proteins that act together to activate Sex-lethal (Sxl) in XX animals. The resulting SXL female specific RNA binding protein modulates the expression of a set of downstream genes, ultimately leading to sexually dimorphic structures and behaviors. Despite the apparent simplicity of this mechanism, Sxl activity is controlled by a host of transcriptional and posttranscriptional mechanisms that tailor its function to specific developmental scenarios. This review describes recent advances in our understanding of Sxl regulation and function, highlighting work that challenges some of the textbook views about this classical (often cited, yet poorly understood) binary switch gene.
Figures



Similar articles
-
Drosophila JAK/STAT pathway reveals distinct initiation and reinforcement steps in early transcription of Sxl.Curr Biol. 2007 Apr 3;17(7):643-8. doi: 10.1016/j.cub.2007.02.038. Epub 2007 Mar 15. Curr Biol. 2007. PMID: 17363251
-
Functional conservation of the sex-lethal sex determining promoter, Sxl-Pe, in Drosophila virilis.Dev Genes Evol. 2003 May;213(4):155-65. doi: 10.1007/s00427-003-0304-1. Epub 2003 Feb 12. Dev Genes Evol. 2003. PMID: 12739140
-
Induction of female Sex-lethal RNA splicing in male germ cells: implications for Drosophila germline sex determination.Development. 1997 Dec;124(24):5033-48. doi: 10.1242/dev.124.24.5033. Development. 1997. PMID: 9362474
-
RNA binding protein sex-lethal (Sxl) and control of Drosophila sex determination and dosage compensation.Microbiol Mol Biol Rev. 2003 Sep;67(3):343-59, table of contents. doi: 10.1128/MMBR.67.3.343-359.2003. Microbiol Mol Biol Rev. 2003. PMID: 12966139 Free PMC article. Review.
-
Promiscuity in post-transcriptional control of gene expression: Drosophila sex-lethal and its regulatory partnerships.FEBS Lett. 2017 Jun;591(11):1471-1488. doi: 10.1002/1873-3468.12652. Epub 2017 Apr 28. FEBS Lett. 2017. PMID: 28391641 Free PMC article. Review.
Cited by
-
Role of Hakai in m6A modification pathway in Drosophila.Nat Commun. 2021 Apr 12;12(1):2159. doi: 10.1038/s41467-021-22424-5. Nat Commun. 2021. PMID: 33846330 Free PMC article.
-
Molecular evolution of Drosophila Sex-lethal and related sex determining genes.BMC Evol Biol. 2012 Jan 14;12:5. doi: 10.1186/1471-2148-12-5. BMC Evol Biol. 2012. PMID: 22244243 Free PMC article.
-
The Influence of Chromosomal Environment on X-Linked Gene Expression in Drosophila melanogaster.Genome Biol Evol. 2020 Dec 6;12(12):2391-2402. doi: 10.1093/gbe/evaa227. Genome Biol Evol. 2020. PMID: 33104185 Free PMC article.
-
Drosophila as a Model System for Studying of the Evolution and Functional Specialization of the Y Chromosome.Int J Mol Sci. 2022 Apr 10;23(8):4184. doi: 10.3390/ijms23084184. Int J Mol Sci. 2022. PMID: 35457001 Free PMC article. Review.
-
PPS, a large multidomain protein, functions with sex-lethal to regulate alternative splicing in Drosophila.PLoS Genet. 2010 Mar 5;6(3):e1000872. doi: 10.1371/journal.pgen.1000872. PLoS Genet. 2010. PMID: 20221253 Free PMC article.
References
-
- Bell LR, Maine EM, Schedl P, Cline TW. Sex-lethal, a Drosophila sex determination switch gene, exhibits sex-specific RNA splicing and sequence similarity to RNA binding proteins. Cell. 1988;55:1037–46. - PubMed
-
- Salz HK, Maine EM, Keyes LN, Samuels ME, Cline TW, Schedl P. The Drosophila female-specific sex determination gene, Sex-lethal, has stage-, tissue- and sex-specific RNAs suggesting multiple modes of regulation. Genes Dev. 1989;3:708–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
