Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis
- PMID: 20160716
- PMCID: PMC3135002
- DOI: 10.1038/nbt.1606
Nutrient-sensitized screening for drugs that shift energy metabolism from mitochondrial respiration to glycolysis
Abstract
Most cells have the inherent capacity to shift their reliance on glycolysis relative to oxidative metabolism, and studies in model systems have shown that targeting such shifts may be useful in treating or preventing a variety of diseases ranging from cancer to ischemic injury. However, we currently have a limited number of mechanistically distinct classes of drugs that alter the relative activities of these two pathways. We screen for such compounds by scoring the ability of >3,500 small molecules to selectively impair growth and viability of human fibroblasts in media containing either galactose or glucose as the sole sugar source. We identify several clinically used drugs never linked to energy metabolism, including the antiemetic meclizine, which attenuates mitochondrial respiration through a mechanism distinct from that of canonical inhibitors. We further show that meclizine pretreatment confers cardioprotection and neuroprotection against ischemia-reperfusion injury in murine models. Nutrient-sensitized screening may provide a useful framework for understanding gene function and drug action within the context of energy metabolism.
Conflict of interest statement
Figures



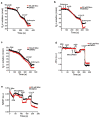
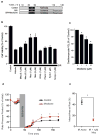
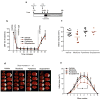
Comment in
-
Drug screening: Shifting energy metabolism.Nat Rev Drug Discov. 2010 Apr;9(4):272. doi: 10.1038/nrd3146. Nat Rev Drug Discov. 2010. PMID: 20535845 No abstract available.
References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Bonnet S, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. - PubMed
-
- Huber R, Spiegel T, Buchner M, Riepe MW. Graded reoxygenation with chemical inhibition of oxidative phosphorylation improves posthypoxic recovery in murine hippocampal slices. J Neurosci Res. 2004;75:441–449. - PubMed
-
- Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

