MutSbeta exceeds MutSalpha in dinucleotide loop repair
- PMID: 20160730
- PMCID: PMC2844022
- DOI: 10.1038/sj.bjc.6605531
MutSbeta exceeds MutSalpha in dinucleotide loop repair
Abstract
Background: The target substrates of DNA mismatch recognising factors MutSalpha (MSH2+MSH6) and MutSbeta (MSH2+MSH3) have already been widely researched. However, the extent of their functional redundancy and clinical substance remains unclear. Mismatch repair (MMR)-deficient tumours are strongly associated with microsatellite instability (MSI) and the degree and type of MSI seem to be dependent on the MMR gene affected, and is linked to its substrate specificities. Deficiency in MSH2 and MSH6 is associated with both mononucleotide and dinucleotide repeat instability. Although no pathogenic MSH3 mutations have been reported, its deficiency is also suggested to cause low dinucleotide repeat instability.
Methods: To assess the substrate specificities and functionality of MutSalpha and MutSbeta we performed an in vitro MMR assay using three substrate constructs, GT mismatch, 1 and 2 nucleotide insertion/deletion loops (IDLs) in three different cell lines.
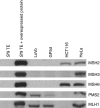
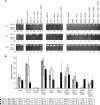
Results: Our results show that though MutSalpha alone seems to be responsible for GT and IDL1 repair, MutSalpha and MutSbeta indeed have functional redundancy in IDL2 repair and in contrast with earlier studies, MutSbeta seems to exceed MutSalpha.
Conclusion: The finding is clinically relevant because the strong role of MutSbeta in IDL2 repair indicates MSH3 deficiency in tumours with low dinucleotide and no mononucleotide repeat instability.
Figures

Similar articles
-
A MutSβ-Dependent Contribution of MutSα to Repeat Expansions in Fragile X Premutation Mice?PLoS Genet. 2016 Jul 18;12(7):e1006190. doi: 10.1371/journal.pgen.1006190. eCollection 2016 Jul. PLoS Genet. 2016. PMID: 27427765 Free PMC article.
-
Schizosaccharomyces pombe MutSα and MutLα Maintain Stability of Tetra-Nucleotide Repeats and Msh3 of Hepta-Nucleotide Repeats.G3 (Bethesda). 2017 May 5;7(5):1463-1473. doi: 10.1534/g3.117.040816. G3 (Bethesda). 2017. PMID: 28341698 Free PMC article.
-
Differential mismatch recognition specificities of eukaryotic MutS homologs, MutSα and MutSβ.Biophys J. 2014 Jun 3;106(11):2483-92. doi: 10.1016/j.bpj.2014.04.026. Biophys J. 2014. PMID: 24896128 Free PMC article.
-
Disease-associated repeat instability and mismatch repair.DNA Repair (Amst). 2016 Feb;38:117-126. doi: 10.1016/j.dnarep.2015.11.008. Epub 2015 Dec 12. DNA Repair (Amst). 2016. PMID: 26774442 Review.
-
Genetic and genomic basis of the mismatch repair system involved in Lynch syndrome.Int J Clin Oncol. 2019 Sep;24(9):999-1011. doi: 10.1007/s10147-019-01494-y. Epub 2019 Jul 4. Int J Clin Oncol. 2019. PMID: 31273487 Review.
Cited by
-
PMS2 expression decrease causes severe problems in mismatch repair.Hum Mutat. 2019 Jul;40(7):904-907. doi: 10.1002/humu.23756. Epub 2019 Apr 18. Hum Mutat. 2019. PMID: 30946512 Free PMC article.
-
Tumor-specific microsatellite instability: do distinct mechanisms underlie the MSI-L and EMAST phenotypes?Mutat Res. 2013 Mar-Apr;743-744:67-77. doi: 10.1016/j.mrfmmm.2012.11.003. Epub 2012 Dec 1. Mutat Res. 2013. PMID: 23206442 Free PMC article. Review.
-
Immunological Features with DNA Microsatellite Alterations in Patients with Colorectal Cancer.J Cancer Immunol (Wilmington). 2020;2(3):116-127. doi: 10.33696/cancerimmunol.2.024. J Cancer Immunol (Wilmington). 2020. PMID: 33000102 Free PMC article.
-
EMAST Type of Microsatellite Instability-A Distinct Entity or Blurred Overlap between Stable and MSI Tumors.Genes (Basel). 2023 Jul 19;14(7):1474. doi: 10.3390/genes14071474. Genes (Basel). 2023. PMID: 37510378 Free PMC article. Review.
-
Inflammation-associated microsatellite alterations: Mechanisms and significance in the prognosis of patients with colorectal cancer.World J Gastrointest Oncol. 2018 Jan 15;10(1):1-14. doi: 10.4251/wjgo.v10.i1.1. World J Gastrointest Oncol. 2018. PMID: 29375743 Free PMC article. Review.
References
-
- Aaltonen LA, Peltomäki P, Leach FS, Sistonen P, Pylkkänen L, Mecklin JP, Järvinen H, Powell SM, Jen J, Hamilton SR, Petersen GM, Kinzler KW, Vogelstein B, de la Chapelle A (1993) Clues to the pathogenesis of familial colorectal cancer. Science 260: 812–816 - PubMed
-
- Bhattacharyya NP, Ganesh A, Phear G, Richards B, Skandalis A, Meuth M (1995) Molecular analysis of mutations in mutator colorectal carcinoma cell lines. Hum Mol Genet 4: 2057–2064 - PubMed
-
- Cannavo E, Marra G, Sabates-Bellver J, Menigatti M, Lipkin SM, Fischer F, Cejka P, Jiricny J (2005) Expression of the MutL homologue hMLH3 in human cells and its role in DNA mismatch repair. Cancer Res 65: 10759–10766 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

