Population pharmacokinetics of the humanised monoclonal antibody, HuHMFG1 (AS1402), derived from a phase I study on breast cancer
- PMID: 20160731
- PMCID: PMC2833251
- DOI: 10.1038/sj.bjc.6605560
Population pharmacokinetics of the humanised monoclonal antibody, HuHMFG1 (AS1402), derived from a phase I study on breast cancer
Abstract
Background: HuHMFG1 (AS1402) is a humanised monoclonal antibody that has undergone a phase I trial in metastatic breast cancer. The aim of this study was to characterise the pharmacokinetics (PKs) of HuHMFG1 using a population PK model.
Method: Data were derived from a phase I study of 26 patients receiving HuHMFG1 at doses ranging from 1 to 16 mg kg(-1). Data were analysed using NONMEM software and covariates were included. A limited sampling strategy (LSS) was developed using training and a validation data set.
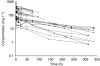
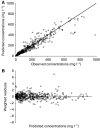
Results: A linear two-compartment model was shown to be adequate to describe data. Covariate analysis indicated that weight was not related to clearance. An LSS was successfully developed on the basis of the model, in which one sample is collected immediately before the start of an infusion and the second is taken at the end of infusion.
Conclusion: A two-compartment population PK model successfully describes HuHMFG1 behaviour. The model suggests using a fixed dose of HuHMFG1, which would simplify dosing. The model could be used to optimise dose level and dosing schedule if more data on the correlation between exposure and efficacy become available from future studies. The derived LSS could optimise further PK assessment of this antibody.
Figures


Similar articles
-
Phase I dose escalation pharmacokinetic assessment of intravenous humanized anti-MUC1 antibody AS1402 in patients with advanced breast cancer.Breast Cancer Res. 2009;11(5):R73. doi: 10.1186/bcr2409. Breast Cancer Res. 2009. PMID: 19811637 Free PMC article. Clinical Trial.
-
Rationale for fixed dosing of pertuzumab in cancer patients based on population pharmacokinetic analysis.Pharm Res. 2006 Jun;23(6):1275-84. doi: 10.1007/s11095-006-0205-x. Epub 2006 May 26. Pharm Res. 2006. PMID: 16715358
-
Population pharmacokinetic data analysis of three phase I studies of matuzumab, a humanised anti-EGFR monoclonal antibody in clinical cancer development.Br J Cancer. 2008 Mar 11;98(5):900-6. doi: 10.1038/sj.bjc.6604265. Epub 2008 Mar 4. Br J Cancer. 2008. PMID: 18319714 Free PMC article.
-
Pharmacokinetics of alemtuzumab and the relevance in clinical practice.Leuk Lymphoma. 2008 Dec;49(12):2256-62. doi: 10.1080/10428190802475303. Leuk Lymphoma. 2008. PMID: 19052972 Review.
-
Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies.Clin Ther. 2006 Nov;28(11):1779-802. doi: 10.1016/j.clinthera.2006.11.015. Clin Ther. 2006. PMID: 17212999 Review.
Cited by
-
Modeling the pharmacokinetic-pharmacodynamic relationship of the monoclonal anti-macaque-IL-15 antibody Hu714MuXHu in cynomolgus monkeys.Pharmacol Res Perspect. 2016 Jan 11;3(6):e00199. doi: 10.1002/prp2.199. eCollection 2015 Dec. Pharmacol Res Perspect. 2016. PMID: 27022472 Free PMC article.
-
Population PK/PD analysis of metformin using the signal transduction model.Br J Clin Pharmacol. 2012 Nov;74(5):815-23. doi: 10.1111/j.1365-2125.2012.04260.x. Br J Clin Pharmacol. 2012. PMID: 22380769 Free PMC article. Clinical Trial.
-
Pharmacokinetics and Biodistribution of a [89Zr]Zr-DFO-MSTP2109A Anti-STEAP1 Antibody in Metastatic Castration-Resistant Prostate Cancer Patients.Mol Pharm. 2019 Jul 1;16(7):3083-3090. doi: 10.1021/acs.molpharmaceut.9b00326. Epub 2019 May 31. Mol Pharm. 2019. PMID: 31117485 Free PMC article.
-
Upregulation of mucin glycoprotein MUC1 in the progression to esophageal adenocarcinoma and therapeutic potential with a targeted photoactive antibody-drug conjugate.Oncotarget. 2017 Apr 11;8(15):25080-25096. doi: 10.18632/oncotarget.15340. Oncotarget. 2017. PMID: 28212575 Free PMC article.
-
Transcriptome- and proteome-wide association studies nominate determinants of kidney function and damage.Genome Biol. 2023 Jun 26;24(1):150. doi: 10.1186/s13059-023-02993-y. Genome Biol. 2023. PMID: 37365616 Free PMC article.
References
-
- Beal SL, Sheiner LB, Boeckmann AJ (eds) (1989–2006) NONMEM users guide (1989–2008). Icon Development Solutions: Ellicott City, MD
-
- Bruno R, Washington CB, Lu JF, Lieberman G, Banken L, Klein P (2005) Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol 56: 361–369 - PubMed
-
- Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41 - PubMed
-
- Comets E, Brendel K, Mentre F (2008) Computing normalised prediction distribution errors to evaluate nonlinear mixed-effect models: the npde add-on package for R. Comput Methods Programs Biomed 90: 154–166 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

