Platinum(II) Enyne Cycloisomerization Catalysis: Intermediates and Resting States
- PMID: 20160853
- PMCID: PMC2662608
- DOI: 10.1021/om801069s
Platinum(II) Enyne Cycloisomerization Catalysis: Intermediates and Resting States
Abstract
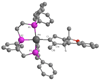
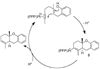
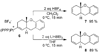
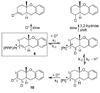
In situ generated [(PPP)Pt][BF(4)](2) (PPP = triphos) catalyzes the cycloisomerization of 1,6-enyne-ols by initiative pi-activation of the alkyne. This generates an isolable cationic Pt-alkenyl species which subsequently participates in turnover limiting protonolysis with in situ generated acid. This latter reactivity contrasts cationic Pt-alkyls which are more difficult to protonolyze. Mechanistic studies on isolated Pt-alkenyls, and deuterium labeling helped to elucidate the mechanistic details.
Figures
Similar articles
-
Computational Analysis of Diastereoselectivity and Carbene Reactivity in Pt- and Au-Catalyzed 1,5-Enyne Cycloisomerization to Bicyclo[3.1.0]hexane.J Org Chem. 2025 Mar 21;90(11):3834-3840. doi: 10.1021/acs.joc.4c02626. Epub 2025 Feb 10. J Org Chem. 2025. PMID: 39928988
-
Platinum-catalyzed cycloisomerization reactions of enynes.J Am Chem Soc. 2001 Dec 5;123(48):11863-9. doi: 10.1021/ja0109343. J Am Chem Soc. 2001. PMID: 11724592
-
Promoting C-C Bond Coupling of Benzyne and Methyl Ligands in Electron-Deficient (triphos)Pt-CH3+ Complexes.Organometallics. 2015 Jun 22;34(12):2707-2709. doi: 10.1021/acs.organomet.5b00121. Epub 2015 Jun 2. Organometallics. 2015. PMID: 26146438 Free PMC article.
-
Electrophilic activation of alkynes for enyne cycloisomerization reactions with in situ generated early/late heterobimetallic Pt-Ti catalysts.Dalton Trans. 2016 Jun 14;45(24):9770-3. doi: 10.1039/c6dt01783e. Dalton Trans. 2016. PMID: 27240482
-
[Development of deuterium labeling method based on the heterogeneous platinum group metal-catalyzed C-H activation].Yakugaku Zasshi. 2013;133(11):1177-93. doi: 10.1248/yakushi.13-00218. Yakugaku Zasshi. 2013. PMID: 24189559 Review. Japanese.
Cited by
-
Gold(I)-catalyzed cascade cyclization of allenyl epoxides.Org Lett. 2009 Aug 6;11(15):3490-2. doi: 10.1021/ol901391s. Org Lett. 2009. PMID: 19588972 Free PMC article.
-
Chemoenzymatic Generation of Thio-analogues of δ-Cadinene, δ-Cadinol, and a Thio-diquinane Using 8-Thio-farnesylpyrophosphate.J Nat Prod. 2025 Jul 25;88(7):1653-1662. doi: 10.1021/acs.jnatprod.5c00409. Epub 2025 Jul 17. J Nat Prod. 2025. PMID: 40673503 Free PMC article.
-
Exploration of cascade cyclizations terminated by tandem aromatic substitution: total synthesis of (+)-schweinfurthin A.J Org Chem. 2011 Feb 4;76(3):909-19. doi: 10.1021/jo1022102. Epub 2011 Jan 12. J Org Chem. 2011. PMID: 21226493 Free PMC article.
-
Synthesis of a Novel Type of 2,3'-BIMs via Platinum-Catalysed Reaction of Indolylallenes with Indoles.Chemistry. 2018 Apr 20;24(23):6105-6114. doi: 10.1002/chem.201705417. Epub 2018 Mar 5. Chemistry. 2018. PMID: 29393548 Free PMC article.
-
Enantioselective Iridium-Catalyzed Allylation of Acetylenic Ketones via 2-Propanol-Mediated Reductive Coupling of Allyl Acetate: C14-C23 of Pladienolide D.Angew Chem Int Ed Engl. 2019 Dec 19;58(52):18803-18807. doi: 10.1002/anie.201908939. Epub 2019 Nov 7. Angew Chem Int Ed Engl. 2019. PMID: 31490591 Free PMC article.
References
-
- Chianese AR, Lee SJ, Gagné MR. Angew. Chem., Int. Ed. 2007;46:4042–4059. - PubMed
-
-
For references showing the stability of cationic Pt-alkyls to protonolysis see: Heyduk AF, Labinger JA, Bercaw JE. J. Am. Chem. Soc. 2003;125:6366–6367. Thorn DL. Organometallics. 1998;17:348–352. Annibale G, Bergamini P, Cattabriga M. Inorg. Chim. Acta. 2001;316:25–32. Butikofer JL, Hoerter JM, Peters RG, Roddick DM. Organometallics. 2004;23:400–408. Peters RG, White S, Roddick DM. Organometallics. 1998;17:4493–4499.
-
-
- Stewart R, Dolman D. Can. J. Chem. 1967;45:925–928.
-
- Feducia JA, Campbell AN, Anthis JW, Gagné MR. Organometallics. 2006;25:3114–3117.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
