Platinum(II) Enyne Cycloisomerization Catalysis: Intermediates and Resting States
- PMID: 20160853
- PMCID: PMC2662608
- DOI: 10.1021/om801069s
Platinum(II) Enyne Cycloisomerization Catalysis: Intermediates and Resting States
Abstract
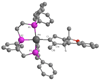
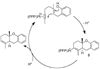
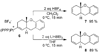
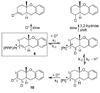
In situ generated [(PPP)Pt][BF(4)](2) (PPP = triphos) catalyzes the cycloisomerization of 1,6-enyne-ols by initiative pi-activation of the alkyne. This generates an isolable cationic Pt-alkenyl species which subsequently participates in turnover limiting protonolysis with in situ generated acid. This latter reactivity contrasts cationic Pt-alkyls which are more difficult to protonolyze. Mechanistic studies on isolated Pt-alkenyls, and deuterium labeling helped to elucidate the mechanistic details.
Figures
References
-
- Chianese AR, Lee SJ, Gagné MR. Angew. Chem., Int. Ed. 2007;46:4042–4059. - PubMed
-
-
For references showing the stability of cationic Pt-alkyls to protonolysis see: Heyduk AF, Labinger JA, Bercaw JE. J. Am. Chem. Soc. 2003;125:6366–6367. Thorn DL. Organometallics. 1998;17:348–352. Annibale G, Bergamini P, Cattabriga M. Inorg. Chim. Acta. 2001;316:25–32. Butikofer JL, Hoerter JM, Peters RG, Roddick DM. Organometallics. 2004;23:400–408. Peters RG, White S, Roddick DM. Organometallics. 1998;17:4493–4499.
-
-
- Stewart R, Dolman D. Can. J. Chem. 1967;45:925–928.
-
- Feducia JA, Campbell AN, Anthis JW, Gagné MR. Organometallics. 2006;25:3114–3117.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
