An Evaluation of Explicit Receptor Flexibility in Molecular Docking Using Molecular Dynamics and Torsion Angle Molecular Dynamics
- PMID: 20160879
- PMCID: PMC2772076
- DOI: 10.1021/ct900262t
An Evaluation of Explicit Receptor Flexibility in Molecular Docking Using Molecular Dynamics and Torsion Angle Molecular Dynamics
Abstract
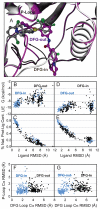
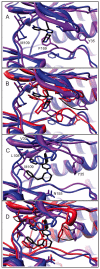
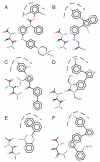
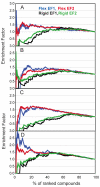
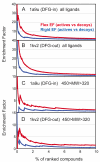
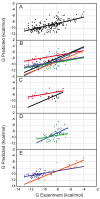
Incorporating receptor flexibility into molecular docking should improve results for flexible proteins. However, the incorporation of explicit all-atom flexibility with molecular dynamics for the entire protein chain may also introduce significant error and "noise" that could decrease docking accuracy and deteriorate the ability of a scoring function to rank native-like poses. We address this apparent paradox by comparing the success of several flexible receptor models in cross-docking and multiple receptor ensemble docking for p38α mitogen-activated protein (MAP) kinase. Explicit all-atom receptor flexibility has been incorporated into a CHARMM-based molecular docking method (CDOCKER) using both molecular dynamics (MD) and torsion angle molecular dynamics (TAMD) for the refinement of predicted protein-ligand binding geometries. These flexible receptor models have been evaluated, and the accuracy and efficiency of TAMD sampling is directly compared to MD sampling. Several flexible receptor models are compared, encompassing flexible side chains, flexible loops, multiple flexible backbone segments, and treatment of the entire chain as flexible. We find that although including side chain and some backbone flexibility is required for improved docking accuracy as expected, docking accuracy also diminishes as additional and unnecessary receptor flexibility is included into the conformational search space. Ensemble docking results demonstrate that including protein flexibility leads to to improved agreement with binding data for 227 active compounds. This comparison also demonstrates that a flexible receptor model enriches high affinity compound identification without significantly increasing the number of false positives from low affinity compounds.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
