Spontaneous onset of atrial fibrillation
- PMID: 20160895
- PMCID: PMC2768313
- DOI: 10.1016/j.physd.2008.12.004
Spontaneous onset of atrial fibrillation
Abstract
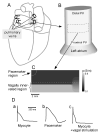
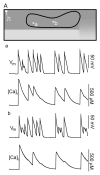
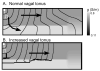
Most commonly, atrial fibrillation is triggered by rapid bursts of electrical impulses originating in the myocardial sleeves of pulmonary veins (PVs). However, the nature of such bursts remains poorly understood. Here, we propose a mechanism of bursting consistent with the extensive empirical information about the electrophysiology of the PVs. The mechanism is essentially non-local and involves the spontaneous initiation of non-sustained spiral waves in the distal end of the muscle sleeves of the PVs. It reproduces the experimentally observed dynamics of the bursts, including their frequency, their intermittent character, and the unusual shape of the electrical signals in the pulmonary veins that are reminiscent of so-called early afterdepolarizations (EADs).
Figures



Similar articles
-
Spontaneous pulmonary vein firing in man: relationship to tachycardia-pause early afterdepolarizations and triggered arrhythmia in canine pulmonary veins in vitro.J Cardiovasc Electrophysiol. 2007 Sep;18(10):1067-75. doi: 10.1111/j.1540-8167.2007.00909.x. Epub 2007 Jul 26. J Cardiovasc Electrophysiol. 2007. PMID: 17655663
-
Myocardial sleeves of pulmonary veins and atrial fibrillation: a postmortem histopathological study of 100 subjects.Virchows Arch. 2006 Jul;449(1):88-95. doi: 10.1007/s00428-006-0197-2. Epub 2006 Apr 13. Virchows Arch. 2006. PMID: 16612621
-
Increased extracellular collagen matrix in myocardial sleeves of pulmonary veins: an additional mechanism facilitating repetitive rapid activities in chronic pacing-induced sustained atrial fibrillation.J Cardiovasc Electrophysiol. 2005 Jul;16(7):753-9. doi: 10.1046/j.1540-8167.2005.40794.x. J Cardiovasc Electrophysiol. 2005. PMID: 16050834
-
Experimental evaluation of the cardiac rhythm originating in myocardial sleeves of pulmonary veins using a monophasic action potential.Physiol Res. 2013;62(Suppl 1):S49-56. doi: 10.33549/physiolres.932604. Physiol Res. 2013. PMID: 24329703 Review.
-
[Modern conceptions of the atrial fibrillation development. The role of the myocardial sleeves in the pulmonary veins].Usp Fiziol Nauk. 2010 Oct-Dec;41(4):3-26. Usp Fiziol Nauk. 2010. PMID: 21254540 Review. Russian.
Cited by
-
A two layers monodomain model of cardiac electrophysiology of the atria.J Math Biol. 2015 Dec;71(6-7):1607-41. doi: 10.1007/s00285-015-0861-8. Epub 2015 Mar 15. J Math Biol. 2015. PMID: 25773466
-
Early afterdepolarizations promote transmural reentry in ischemic human ventricles with reduced repolarization reserve.Prog Biophys Mol Biol. 2016 Jan;120(1-3):236-48. doi: 10.1016/j.pbiomolbio.2016.01.008. Epub 2016 Feb 2. Prog Biophys Mol Biol. 2016. PMID: 26850675 Free PMC article.
-
A simple model of the right atrium of the human heart with the sinoatrial and atrioventricular nodes included.J Clin Monit Comput. 2013 Aug;27(4):481-98. doi: 10.1007/s10877-013-9429-6. Epub 2013 Feb 22. J Clin Monit Comput. 2013. PMID: 23430363 Free PMC article.
-
Fast propagation regions cause self-sustained reentry in excitable media.Proc Natl Acad Sci U S A. 2017 Feb 7;114(6):1281-1286. doi: 10.1073/pnas.1611475114. Epub 2017 Jan 25. Proc Natl Acad Sci U S A. 2017. PMID: 28123066 Free PMC article.
-
Conditions for Waveblock Due to Anisotropy in a Model of Human Ventricular Tissue.PLoS One. 2015 Nov 2;10(11):e0141832. doi: 10.1371/journal.pone.0141832. eCollection 2015. PLoS One. 2015. PMID: 26523734 Free PMC article.
References
-
- Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. - PubMed
-
- Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, Camm J, Akhtar M, Luderitz B. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36:1303–9. - PubMed
-
- Prystowsky EN, Benson DW, Fuster V, Hart RG, Kay GN, Myerburg RJ, Naccarelli GV, Wyse DG. Management of patients with atrial fibrillation. A Statement for Healthcare Professionals. From the Subcommittee on Electrocardiography and Electrophysiology, American Heart Association. Circulation. 1996;93:1262–77. - PubMed
-
- Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. - PubMed
-
- Saksena S, Hettrick DA, Koehler JL, Grammatico A, Padeletti L. Progression of paroxysmal atrial fibrillation to persistent atrial fibrillation in patients with bradyarrhythmias. Am Heart J. 2007;154:884–92. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
