PEI-PEG-Chitosan Copolymer Coated Iron Oxide Nanoparticles for Safe Gene Delivery: synthesis, complexation, and transfection
- PMID: 20160995
- PMCID: PMC2756666
- DOI: 10.1002/adfm.200801844
PEI-PEG-Chitosan Copolymer Coated Iron Oxide Nanoparticles for Safe Gene Delivery: synthesis, complexation, and transfection
Abstract
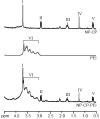
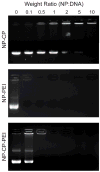
Gene therapy offers the potential of mediating disease through modification of specific cellular functions of target cells. However, effective transport of nucleic acids to target cells with minimal side effects remains a challenge despite the use of unique viral and non-viral delivery approaches. Here we present a non-viral nanoparticle gene carrier that demonstrates effective gene delivery and transfection both in vitro and in vivo. The nanoparticle system (NP-CP-PEI) is made of a superparamagnetic iron oxide nanoparticle (NP), which enables magnetic resonance imaging, coated with a novel copolymer (CP-PEI) comprised of short chain polyethylenimine (PEI) and poly(ethylene glycol) (PEG) grafted to the natural polysaccharide, chitosan (CP), which allows efficient loading and protection of the nucleic acids. The function of each component material in this nanoparticle system is illustrated by comparative studies of three nanoparticle systems of different surface chemistries, through material property characterization, DNA loading and transfection analyses, and toxicity assessment. Significantly, NP-CP-PEI demonstrates an innocuous toxic profile and a high level of expression of the delivered plasmid DNA in a C6 xenograft mouse model, making it a potential candidate for safe in vivo delivery of DNA for gene therapy.
Figures










References
-
- Alexander BL, Ali RR, Alton EW, Bainbridge JW, Braun S, Cheng SH, Flotte TR, Gaspar HB, Grez M, Griesenbach U, Kaplitt MG, Ott MG, Seger R, Simons M, Thrasher AJ, Thrasher AZ, Yla-Herttuala S. Gene Ther. 2007;14:1439. - PubMed
-
- Aiuti A, Bachoud-Levi AC, Blesch A, Brenner MK, Cattaneo F, Chiocca EA, Gao G, High KA, Leen AM, Lemoine NR, McNeish IA, Meneguzzi G, Peschanski M, Roncarolo MG, Strayer DS, Tuszynski MH, Waxman DJ, Wilson JM. Gene Ther. 2007;14:1555. - PubMed
-
- Edelstein ML, Abedi MR, Wixon J. J Gene Med. 2007;9:833. - PubMed
-
- Pack DW, Hoffman AS, Pun S, Stayton PS. Nat Rev Drug Discov. 2005;4:581. - PubMed
-
- McCormack MP, Rabbitts TH. N Engl J Med. 2004;350:913. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
