Induced pluripotent reprogramming from promiscuous human stemness related factors
- PMID: 20161095
- PMCID: PMC2745150
- DOI: 10.1111/j.1752-8062.2009.00091.x
Induced pluripotent reprogramming from promiscuous human stemness related factors
Abstract
Ectopic expression of pluripotency gene sets provokes nuclear reprogramming in permissive somatic tissue environments generating induced pluripotent stem (iPS) cells. The evolutionary conserved function of stemness orthologs was here tested through interspecies transduction. A spectrum of HIV-based lentiviral vectors was designed, and point mutations in the HIV-1 capsid region identified for efficient infectivity and expanded trans-species tropism. Human pluripotent gene sequences, OCT3/4, SOX2, KLF4 and c-MYC, packaged into engineered lentiviral expression vectors achieved consistent expression in non-human fibroblasts. Despite variation in primary amino-acid sequence between species, introduction of human pluripotent genes produced cell lines with embryonic stem cell-like morphology. Transduced fibroblasts differentiated in vitro into all three germ layers according to gastrulation gene expression profiles, and formed in vivo teratoma with multi-lineage potential. Reprogrammed progeny incorporated into non-human morula to produce blastomeres capable of developing into chimeric embryos with competent organogenesis. This model system establishes a prototypic approach to examine consequences of human stemness factors induced reprogramming in the context of normal embryonic development, exploiting non-human early stage embryos. Thus, ectopic xeno-transduction across species unmasks the promiscuous nature of stemness induction, suggesting evolutionary selection of core processes for somatic tissue reprogramming.
Keywords: HIV; KLF4; OCT3/4; SOX2; c-MYC; chimera; iPS; lentiviral; ortholog.
Figures

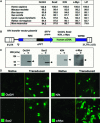
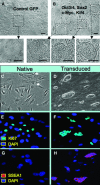
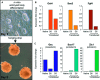
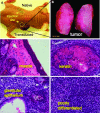

References
-
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997; 385(6691): 810–813. - PubMed
-
- Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996; 380(6569): 64–66. - PubMed
-
- Hochedlinger K, Jaenisch R. Nuclear reprogramming and pluripotency. Nature. 2006; 441(7097): 1061–1067. - PubMed
-
- Armstrong L, Lako M, Dean W, Stojkovic M. Epigenetic modification is central to genome reprogramming in somatic cell nuclear transfer. Stem Cells. 2006; 24(4): 805–814. - PubMed
-
- Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007; 450(7169): 497–502. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

