Computational Studies on the Pt(II)-Catalyzed Cycloisomerization of 1,6-dienes into Bicyclopropanes: A Mechanistic Quandary Evaluated by DFT
- PMID: 20161262
- PMCID: PMC2700740
- DOI: 10.1021/om800760x
Computational Studies on the Pt(II)-Catalyzed Cycloisomerization of 1,6-dienes into Bicyclopropanes: A Mechanistic Quandary Evaluated by DFT
Abstract
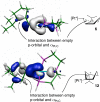
The mechanism of the (bis(phosphanylethyl)phosphane)Pt(2+) catalyzed cyclo-isomerization reaction of 7-methyl-octa-1,6-diene to form 1-isopropylbicyclo[3.1.0]hexane was studied using computational methods. The cyclopropanation step was found to be the turnover-limiting step. The overall reaction proceeds via both a 5-exo and a 6-endo route. W conformations were shown to facilitate cyclopropanation, but do not have any influence on the rate of the 1,2-hydride shifts.
Figures




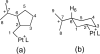

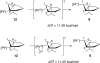




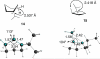

References
-
-
For an entry into metal-catalyzed cycloisomerization reactions, see:Michelet V, Toullec PY, Genet J-P. Angew. Chem. Int. Ed. 2008;47:4268–4315.Diver ST, Giessert AJ. Chem. Rev. 2004;104:1317–1382.Echavarren AM, Nevado C. Chem. Soc. Rev. 2004;33:431–436.Méndez M, Mamane V, Fürstner A. Chemtracts. 2003;16:397–425.Ojima I, Tzamarioudaki M, Li Z, Donovan RJ. Chem. Rev. 1996;96:635–662. For a mechanistic discussion of metal-catalyzed cycloisomerization reactions, see: Lloyd-Jones GC. Org. Biomol. Chem. 2003;1:215–236.
-
-
- Christianson DW. Chem. Rev. 2006;106:3412–3442. - PubMed
- Croteau R. Chem. Rev. 1987;87:929–954.
-
- Yoder RA, Johnston JN. Chem. Rev. 2005;105:4730–4756. - PMC - PubMed
- Wendt KU, Schultz GE, Corey EJ, Liu DR. Angew. Chem. Int. Ed. 2000;39:2812–2833. - PubMed
- Sutherland JK. In: Comprehensive Organic Synthesis. Trost BM, editor. Vol. 1. Pergamon Press; 1991. pp. 341–377.
- Bartlett PA. In: Asymmetric Synthesis. Morrison JD, editor. Vol. 3. Academic Press; New York: 1984. pp. 341–409.
-
- Trost BM, Toste FD, Pinkerton AB. Chem. Rev. 2001;101:2067–2096. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
