An amine-derivatized, DOTA-loaded polymeric support for Fmoc Solid Phase Peptide Synthesis
- PMID: 20161272
- PMCID: PMC2702766
- DOI: 10.1016/j.tetlet.2009.05.061
An amine-derivatized, DOTA-loaded polymeric support for Fmoc Solid Phase Peptide Synthesis
Abstract
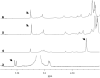
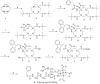
An amine-derivatized DOTA has been used to modify the surface of a polymeric support for conventional Solid Phase Peptide Synthesis (SPPS) following standard Fmoc chemistry methods. This methodology was used to synthesize a peptide-DOTA conjugate that was demonstrated to be a PARACEST MRI contrast agent. Therefore, this synthesis methodology can facilitate Fmoc SPPS of molecular imaging contrast agents.
Figures
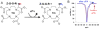
References
-
- Massoudand TF, Gambhir SS. Genes Dev. 2003;17:545. - PubMed
-
- De Leon-Rodriquez LM, Ortiz A, Weiner AL, Zhang S, Kovacs Z, Kodadek T, Sherry AD. J Am Chem Soc. 2002;124:3514. - PubMed
-
- Tung CH. Biopolymers (Pept Sci) 2004;76:391. - PubMed
-
- Knight LC. Handbook of Radiopharmaceuticals: Radiochemistry and Applications. John Wiley and Sons; New York: 2003. pp. 643–684.
-
- Aina OH, Liu R, Sutcliffe JL, Marik J, Pan CX, Lam KS. Mol Pharmaceutics. 2007;4:631. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
