A new approach to carbon-carbon bond formation: Development of aerobic Pd-catalyzed reductive coupling reactions of organometallic reagents and styrenes
- PMID: 20161306
- PMCID: PMC2699303
- DOI: 10.1016/j.tet.2009.03.096
A new approach to carbon-carbon bond formation: Development of aerobic Pd-catalyzed reductive coupling reactions of organometallic reagents and styrenes
Abstract
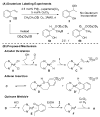
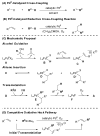
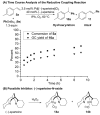
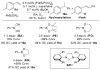
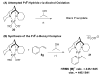
Alkenes are attractive starting materials for organic synthesis and the development of new selective functionalization reactions are desired. Previously, our laboratory discovered a unique Pd-catalyzed hydroalkoxylation reaction of styrenes containing a phenol. Based upon deuterium labeling experiments, a mechanism involving an aerobic alcohol oxidation coupled to alkene functionalization was proposed. These results inspired the development of a new Pd-catalyzed reductive coupling reaction of alkenes and organometallic reagents that generates a new carbon-carbon bond. Optimization of the conditions for the coupling of both organostannanes and organoboronic esters is described and the initial scope of the transformation is presented. Additionally, several mechanistic experiments are outlined and support the rationale for the development of the reaction based upon coupling alcohol oxidation to alkene functionalization.
Figures

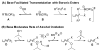
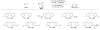
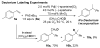

Similar articles
-
Recent Advances in First-Row Transition Metal-Catalyzed Reductive Coupling Reactions for π-Bond Functionalization and C-Glycosylation.Acc Chem Res. 2023 Nov 21;56(22):3292-3312. doi: 10.1021/acs.accounts.3c00531. Epub 2023 Nov 2. Acc Chem Res. 2023. PMID: 37917928
-
Cross-coupling reaction of alkyl halides with grignard reagents catalyzed by Ni, Pd, or Cu complexes with pi-carbon ligand(s).Acc Chem Res. 2008 Nov 18;41(11):1545-54. doi: 10.1021/ar800138a. Acc Chem Res. 2008. PMID: 18973349
-
Palladium(II)-Catalyzed Oxidative Difunctionalization of Alkenes: Bond Forming at a High-Valent Palladium Center.Acc Chem Res. 2016 Nov 15;49(11):2413-2423. doi: 10.1021/acs.accounts.6b00328. Epub 2016 Oct 14. Acc Chem Res. 2016. PMID: 27739689
-
The Progress of Reductive Coupling Reaction by Iron Catalysis.Chem Rec. 2024 Nov;24(11):e202400108. doi: 10.1002/tcr.202400108. Epub 2024 Sep 17. Chem Rec. 2024. PMID: 39289832 Review.
-
Direct functionalization of nitrogen heterocycles via Rh-catalyzed C-H bond activation.Acc Chem Res. 2008 Aug;41(8):1013-25. doi: 10.1021/ar800042p. Epub 2008 Jul 11. Acc Chem Res. 2008. PMID: 18616300 Free PMC article. Review.
Cited by
-
Palladium-catalyzed hydrofunctionalization of vinyl phenol derivatives with heteroaromatics.Org Lett. 2011 May 20;13(10):2774-7. doi: 10.1021/ol200913r. Epub 2011 Apr 21. Org Lett. 2011. PMID: 21510631 Free PMC article.
-
Asymmetric Hydroarylation of Vinylarenes Using a Synergistic Combination of CuH and Pd Catalysis.J Am Chem Soc. 2016 Jul 13;138(27):8372-5. doi: 10.1021/jacs.6b04566. Epub 2016 Jul 1. J Am Chem Soc. 2016. PMID: 27346525 Free PMC article.
-
A Dual Palladium and Copper Hydride Catalyzed Approach for Alkyl-Aryl Cross-Coupling of Aryl Halides and Olefins.Angew Chem Int Ed Engl. 2017 Jun 12;56(25):7242-7246. doi: 10.1002/anie.201703400. Epub 2017 May 16. Angew Chem Int Ed Engl. 2017. PMID: 28510287 Free PMC article.
-
Palladium-catalyzed hydroalkylation of styrenes with organozinc reagents to form carbon-carbon sp3-sp3 bonds under oxidative conditions.J Am Chem Soc. 2009 Dec 23;131(50):18042-3. doi: 10.1021/ja908545b. J Am Chem Soc. 2009. PMID: 19929001 Free PMC article.
-
Pd(0)-catalyzed 1,1-diarylation of ethylene and allylic carbonates.Org Lett. 2013 Oct 4;15(19):5008-11. doi: 10.1021/ol4023358. Epub 2013 Sep 18. Org Lett. 2013. PMID: 24047468 Free PMC article.
References
-
- Beller M, Seayad J, Tillack A, Jiao H. Angew Chem, Int Ed. 2004;43:3368–3398. - PubMed
-
-
Gligorich KM, Schultz MJ, Sigman MS. J Am Chem Soc. 2006;128:2794–2795.Zhang Y, Sigman MS. Org Lett. 2006;8:5557–5560.For an example of a PdII-catalyzed coupled alcohol oxidation/alkene hydrogenation, see: Tsuchiya Y, Hamashima Y, Sodeoka M. Org Lett. 2006;8:4851–4854.
-
-
-
For examples of metal catalyzed alkene hydroalkoxylation reactions, see: Qian H, Han X, Widenhoefer RA. J Am Chem Soc. 2004;126:9536–9537.Yang CG, Reich NW, Shi Z, He C. Org Lett. 2005;7:4553–4556.Coulombel L, Favier I, Dunach E. Chem Commun. 2005:2286–2288.Yang CG, He C. J Am Chem Soc. 2005;127:6966–6967.
-
Grants and funding
LinkOut - more resources
Full Text Sources
