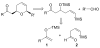Synthesis of 2,6-disubstituted dihydropyrans via an efficient BiBr(3)-initiated three component, one-pot cascade
- PMID: 20161364
- PMCID: PMC2777747
- DOI: 10.1016/j.tet.2009.06.083
Synthesis of 2,6-disubstituted dihydropyrans via an efficient BiBr(3)-initiated three component, one-pot cascade
Abstract
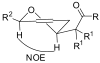
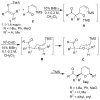
The rapid synthesis of cis-2,6-disubstituted dihydropyrans is achieved in a three-component, one-pot cascade reaction. BiBr(3)-ediated addition of ketene silyl acetals or silyl enol ethers to beta,gamma-unsaturated cis-4-trimethylsilyl-3-butenal provides a Mukaiyama aldol adduct containing a vinylsilane moiety tethered to a silyl ether. Addition of a second aldehyde initiates a domino sequence involving intermolecular addition followed by an intramolecular silyl-modified Sakurai (ISMS) reaction. Isolated yields of this one-pot reaction vary from 44 to 80% and all compounds were isolated as the cis-diastereomers (10 examples).
Figures
Similar articles
-
Stereoselective Sc(OTf)3 -Catalyzed Aldol Reactions of Disubstituted Silyl Enol Ethers of Aldehydes with Acetals.Angew Chem Int Ed Engl. 2021 Jun 1;60(23):12765-12769. doi: 10.1002/anie.202101634. Epub 2021 May 6. Angew Chem Int Ed Engl. 2021. PMID: 33779017
-
Sakurai reaction of 3,3-bis(silyl) silyl enol ethers with acetals involving selective desilylation of the geminal bis(silane). Concise synthesis of nematocidal oxylipid.Org Lett. 2013 Mar 1;15(5):1068-71. doi: 10.1021/ol400069p. Epub 2013 Feb 11. Org Lett. 2013. PMID: 23398287
-
Mixed Monosilyl Acetals and Catalyst-Dependent Chemoselective Mukaiyama Aldol Reactions.Chemistry. 2017 Nov 21;23(65):16432-16437. doi: 10.1002/chem.201704456. Epub 2017 Oct 30. Chemistry. 2017. PMID: 28940890
-
Mukaiyama aldol reaction: an effective asymmetric approach to access chiral natural products and their derivatives/analogues.RSC Adv. 2023 Nov 8;13(47):32975-33027. doi: 10.1039/d3ra05058k. eCollection 2023 Nov 7. RSC Adv. 2023. PMID: 38025859 Free PMC article. Review.
-
The vinylogous Mukaiyama aldol reaction (VMAR) in natural product synthesis.Nat Prod Rep. 2014 Apr;31(4):563-94. doi: 10.1039/c3np70102f. Epub 2014 Mar 5. Nat Prod Rep. 2014. PMID: 24595879 Review.
Cited by
-
Atom economical, one-pot, three-reaction cascade to novel tricyclic 2,4-dihydro-1H-benzo[f]isochromenes.Org Lett. 2013 Aug 16;15(16):4070-3. doi: 10.1021/ol401600j. Epub 2013 Aug 1. Org Lett. 2013. PMID: 23901903 Free PMC article.
-
Bi(OTf)3-, TfOH-, and TMSOTf-mediated, one-pot epoxide rearrangement, addition, and intramolecular silyl-modified Sakurai (ISMS) cascade toward dihydropyrans: comparison of catalysts and role of Bi(OTf)3.J Org Chem. 2011 Nov 18;76(22):9269-77. doi: 10.1021/jo201478d. Epub 2011 Oct 25. J Org Chem. 2011. PMID: 21916500 Free PMC article.
References
-
- Zhu J, Beinaymé H, editors. Multicomponent Reactions. Wiley-VCH; Weinheim: 2005.
-
- Tietze LF, Brasche G, Gericke KM, editors. Domino Reactions in Organic Synthesis. WILEY-VCH; Weinheim: 2006. Multi-step reactions relying upon the formation and conversion of initial reaction products to subsequent compounds have been called tandem reactions, cascade reactions, and, more recently, domino reactions.
-
- Hua R. Curr Org Synth. 2008;5:1–27.
- Komatsu N. In: Organobismuth Chemistry. Suzuki H, Matano Y, editors. Elsevier; New York: 2001. pp. 371–440.
- Gaspard-Iloughmane H, Le Roux C. Eur J Org Chem. 2004;12:2517–2532.
- Leonard NM, Wieland LC, Mohan RS. Tetrahedron. 2002;58:8373–8397.
-
-
The LD50 of BiCl3 is listed as 3334 mg/kg (oral, rat) and 2250 mg/kg (oral, mouse) in the MSDS provided by Sigma-Aldrich Chemical Corp., St. Louis, MO.
-
-
- Hinkle RJ, Lian Y. J Org Chem. 2006;71:7071–7074. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources