Recent Departures in the Synthesis of Peptides and Glycopeptides
- PMID: 20161377
- PMCID: PMC2780351
- DOI: 10.1016/j.tet.2009.09.032
Recent Departures in the Synthesis of Peptides and Glycopeptides
Abstract
In this account, we describe the results of a research program directed to the proposition that chemical synthesis can play a valuable role in identifying biologic level molecules worthy of pharma level development. We recount our journey towards the chemical synthesis of homogeneous erythropoietin, the challenges we encountered, and our efforts to address deficiencies in the current "state of the art" of glycopeptide synthesis. Here we describe new methods for the synthesis of glycopeptides that have emerged from the erythropoietin adventure, including the development of unique C-terminal acyl donors, novel amide bond forming methods, and new ligation and coupling strategies.
Figures
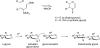
























Similar articles
-
Toward homogeneous erythropoietin: fine tuning of the C-terminal acyl donor in the chemical synthesis of the Cys29-Gly77 glycopeptide domain.J Am Chem Soc. 2009 Apr 22;131(15):5432-7. doi: 10.1021/ja808705v. J Am Chem Soc. 2009. PMID: 20560636 Free PMC article.
-
Toward homogeneous erythropoietin: chemical synthesis of the Ala1-Gly28 glycopeptide domain by "alanine" ligation.J Am Chem Soc. 2009 Apr 22;131(15):5438-43. doi: 10.1021/ja808707w. J Am Chem Soc. 2009. PMID: 19334679 Free PMC article.
-
Solid-phase synthesis of peptide and glycopeptide thioesters through side-chain-anchoring strategies.Chemistry. 2008;14(12):3620-9. doi: 10.1002/chem.200701978. Chemistry. 2008. PMID: 18278777
-
Chemical synthesis of homogeneous glycopeptides and glycoproteins.Chem Rec. 2010 Apr;10(2):80-100. doi: 10.1002/tcr.200900024. Chem Rec. 2010. PMID: 20349507 Review.
-
Recent Development in Ligation Methods for Glycopeptide and Glycoprotein Synthesis.Chem Asian J. 2020 Sep 1;15(17):2548-2557. doi: 10.1002/asia.202000566. Epub 2020 Jul 28. Chem Asian J. 2020. PMID: 32657034 Review.
Cited by
-
Toward Homogeneous Erythropoietin: Application of Metal Free Dethiylation in the Chemical Synthesis of the Ala79-Arg166 Glycopeptide Domain.Isr J Chem. 2011 Nov;51(8-9):968-976. doi: 10.1002/ijch.201100077. Isr J Chem. 2011. PMID: 23585694 Free PMC article.
-
The winding pathway to erythropoietin along the chemistry-biology frontier: a success at last.Angew Chem Int Ed Engl. 2013 Jul 22;52(30):7646-65. doi: 10.1002/anie.201301666. Epub 2013 Jun 17. Angew Chem Int Ed Engl. 2013. PMID: 23775885 Free PMC article. Review.
-
Encouraging progress in the ω-aspartylation of complex oligosaccharides as a general route to β-N-linked glycopolypeptides.J Am Chem Soc. 2011 Feb 9;133(5):1597-602. doi: 10.1021/ja110115a. Epub 2011 Jan 5. J Am Chem Soc. 2011. PMID: 21207981 Free PMC article.
-
Total synthesis of the α-subunit of human glycoprotein hormones: toward fully synthetic homogeneous human follicle-stimulating hormone.J Am Chem Soc. 2012 Feb 22;134(7):3532-41. doi: 10.1021/ja2111459. Epub 2012 Feb 6. J Am Chem Soc. 2012. PMID: 22280541 Free PMC article.
-
Convergent chemical synthesis of [lysine(24,38,83)] human erythropoietin.Angew Chem Int Ed Engl. 2012 Jan 23;51(4):993-9. doi: 10.1002/anie.201106060. Epub 2011 Dec 16. Angew Chem Int Ed Engl. 2012. PMID: 22180156 Free PMC article. No abstract available.
References
-
- Danishefsky SJ, Kerwin JF, Kobayashi S. J. Am. Chem. Soc. 1982;104:358–360.
- Danishefsky SJ, Kato N, Askin D, Kerwin JF. J. Am. Chem. Soc. 1982;104:360–362.
-
- Danishefsky SJ, Kitahara T. J. Am. Chem. Soc. 1974;96:7807–7808.
-
-
For a review, see: Danishefsky SJ, Bilodeau MT. Angew. Chem., Int. Ed. 1996;35:1380–1419.
-
-
-
For a review, see: Seeberger PH, Bilodeau MT, Danishefsky SJ. Aldrichim. Acta. 1997;30:75–92.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
