mGluR4-positive allosteric modulation as potential treatment for Parkinson's disease
- PMID: 20161443
- PMCID: PMC2790174
- DOI: 10.4155/fmc.09.38
mGluR4-positive allosteric modulation as potential treatment for Parkinson's disease
Abstract
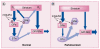
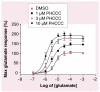
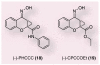
Although Parkinson's disease was first diagnosed nearly 200 years ago, its effective treatment still remains elusive for most of those diagnosed. The gold standard of treatment for most patients is 3,4-dihydroxy-L-phenylalanine. This drug works for most individuals early in the disease; however, resistant symptoms start to emerge after several years of treatment. There has been increased interest in finding novel therapies to help Parkinson's disease patients. Such strategies may have the benefit of not only treating the symptomatic issues of the disorder, but might also offer promise in protecting dopaminergic neurons from further degeneration. One such target that is now receiving much attention from the scientific community is the metabotropic glutamate receptor mGluR4. In this article, we briefly review Parkinson's disease and then recent work in the mGluR area, with a focus on the efforts being made toward finding and optimizing novel mGluR4 positive allosteric modulators (PAMs). Preclinically in rodent models, mGluR4 activation has offered much promise as a novel treatment of Parkinson's disease. Additionally, the specific use of PAMs, rather than direct-acting agonists at the orthosteric glutamate site, continues to be validated as a viable treatment option for this target. It is anticipated that continued progress in this area will further our understanding of the potential of mGluR4 modulation as a novel symptomatic and potentially disease-modifying treatment for Parkinson's disease.
Figures


Similar articles
-
Recent progress in the development of mGluR4 positive allosteric modulators for the treatment of Parkinson's disease.Curr Top Med Chem. 2009;9(10):949-63. Curr Top Med Chem. 2009. PMID: 19754407 Review.
-
mGluR4 positive allosteric modulators with potential for the treatment of Parkinson's disease: WO09010455.Expert Opin Ther Pat. 2010 Mar;20(3):441-5. doi: 10.1517/13543770903551295. Expert Opin Ther Pat. 2010. PMID: 20180624
-
A potent and selective metabotropic glutamate receptor 4 positive allosteric modulator improves movement in rodent models of Parkinson's disease.J Pharmacol Exp Ther. 2012 Oct;343(1):167-77. doi: 10.1124/jpet.112.196063. Epub 2012 Jul 11. J Pharmacol Exp Ther. 2012. PMID: 22787118
-
A case study of foliglurax, the first clinical mGluR4 PAM for symptomatic treatment of Parkinson's disease: translational gaps or a failing industry innovation model?Expert Opin Investig Drugs. 2020 Dec;29(12):1323-1338. doi: 10.1080/13543784.2020.1839047. Epub 2020 Nov 11. Expert Opin Investig Drugs. 2020. PMID: 33074728 Review.
-
Allosteric modulation of group III metabotropic glutamate receptor 4: a potential approach to Parkinson's disease treatment.Proc Natl Acad Sci U S A. 2003 Nov 11;100(23):13668-73. doi: 10.1073/pnas.1835724100. Epub 2003 Oct 30. Proc Natl Acad Sci U S A. 2003. PMID: 14593202 Free PMC article.
Cited by
-
Metabotropic glutamate receptor 4 in the basal ganglia of parkinsonian monkeys: ultrastructural localization and electrophysiological effects of activation in the striatopallidal complex.Neuropharmacology. 2013 Mar;66:242-52. doi: 10.1016/j.neuropharm.2012.05.017. Epub 2012 May 23. Neuropharmacology. 2013. PMID: 22634360 Free PMC article.
-
Solution-phase parallel synthesis and SAR of homopiperazinyl analogs as positive allosteric modulators of mGlu₄.ACS Comb Sci. 2011 Mar 14;13(2):159-65. doi: 10.1021/co1000508. Epub 2011 Feb 21. ACS Comb Sci. 2011. PMID: 21338051 Free PMC article.
-
Neuropharmacological Insight from Allosteric Modulation of mGlu Receptors.Trends Pharmacol Sci. 2019 Apr;40(4):240-252. doi: 10.1016/j.tips.2019.02.006. Epub 2019 Feb 26. Trends Pharmacol Sci. 2019. PMID: 30824180 Free PMC article. Review.
-
Effect of intrahippocampal microinjection of VU0155041, a positive allosteric modulator of mGluR4, on long term potentiation in a valproic acid-induced autistic male rat model.IBRO Neurosci Rep. 2024 May 22;16:629-634. doi: 10.1016/j.ibneur.2024.05.005. eCollection 2024 Jun. IBRO Neurosci Rep. 2024. PMID: 38832089 Free PMC article.
-
Measures of anxiety, sensorimotor function, and memory in male and female mGluR4⁻/⁻ mice.Behav Brain Res. 2012 Apr 1;229(1):21-8. doi: 10.1016/j.bbr.2011.12.037. Epub 2012 Jan 2. Behav Brain Res. 2012. PMID: 22227508 Free PMC article.
References
-
- Dauer W, Predborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39(6):889–909. - PubMed
-
- Schapira AHV. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol. Sci. 2009;30(1):41–47. - PubMed
-
- Hefti FF. Parkinson’s disease. In: Hefti FF, editor. Drug Discovery for Nervous System Diseases. Wiley-Interscience; NJ, USA: 2005. pp. 183–204.
-
- Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann. NY Acad. Sci. 2003;991:1–14. - PubMed
-
-
Parkinson J. An Essay on the Shaking Palsy. Sherwood, Neely and Jones; London, UK: 1817. ■ First report of what is now known as Parkinson’s disease.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
