Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research
- PMID: 20161474
- PMCID: PMC2794050
- DOI: 10.1038/nchem.399
Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research
Abstract
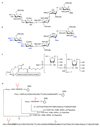
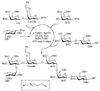
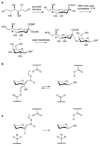
Synthetic oligosaccharides and glycoconjugates are increasingly used as probes for biological research and as lead compounds for drug and vaccine discovery. These endeavors are, however, complicated by a lack of general methods for the routine preparation of this important class of compounds. Recent development such as one-pot multi-step protecting group manipulations, the use of unified monosaccharide building blocks, the introduction of stereoselective glycosylation protocols, and convergent strategies for oligosaccharide assembly, are beginning to address these problems. Furthermore, oligosaccharide synthesis can be facilitated by chemo-enzymatic methods, which employ a range of glycosyl transferases to modify a synthetic oligosaccharide precursor. Glycosynthases, which are mutant glycosidases, that can readily form glycosidic linkages are addressing a lack of a wide range glycosyltransferases. The power of carbohydrate chemistry is highlighted by an ability to synthesize glycoproteins.
Figures



References
-
- Bucior I, Burger MM. Carbohydrate-carbohydrate interactions in cell recognition. Curr. Op. Struct. Biol. 2004;14:631–637. - PubMed
-
- Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. - PubMed
-
- Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. - PubMed
-
- Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446:1030–1037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

