Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro
- PMID: 20161507
- PMCID: PMC2797131
- DOI: 10.1016/j.brs.2009.03.007
Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro
Abstract
Background: The neocortex is the most common target of subdural electrotherapy and noninvasive brain stimulation modalities, including transcranial magnetic stimulation (TMS) and transcranial current simulation (TCS). Specific neuronal elements targeted by cortical stimulation are considered to underlie therapeutic effects, but the exact cell type(s) affected by these methods remains poorly understood.
Objective: We determined whether neuronal morphology or cell type predicted responses to subthreshold and suprathreshold uniform electric fields.
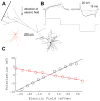
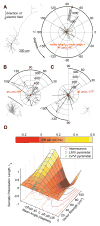
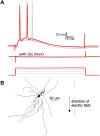
Methods: We characterized the effects of subthreshold and suprathreshold electrical stimulation on identified cortical neurons in vitro. Uniform electric fields were applied to rat motor cortex brain slices, while recording from interneurons and pyramidal cells across cortical layers, using a whole-cell patch clamp. Neuron morphology was reconstructed after intracellular dialysis of biocytin. Based solely on volume-weighted morphology, we developed a parsimonious model of neuronal soma polarization by subthreshold electric fields.
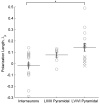
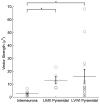
Results: We found that neuronal morphology correlated with somatic subthreshold polarization. Based on neuronal morphology, we predict layer V pyramidal neuronal soma to be individually the most sensitive to polarization by optimally oriented subthreshold fields. Suprathreshold electric field action potential threshold was shown to reflect both direct cell polarization and synaptic (network) activation. Layer V/VI neuron absolute electric field action potential thresholds were lower than layer II/III pyramidal neurons and interneurons. Compared with somatic current injection, electric fields promoted burst firing and modulated action potential firing times.
Conclusions: We present experimental data indicating that cortical neuron morphology relative to electric fields and cortical cell type are factors in determining sensitivity to sub- and supra-threshold brain stimulation.
Figures


References
-
- Liebetanz D, et al. Anticonvulsant effects of transcranial direct-current stimulation (tDCS) in the rat cortical ramp model of focal epilepsy. Epilepsia. 2006;47(7):1216–24. - PubMed
-
- George MS, Lisanby SH, Sackeim HA. Transcranial magnetic stimulation: applications in neuropsychiatry. Arch Gen Psychiatry. 1999;56(4):300–11. - PubMed
-
- Avery DH, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59(2):187–94. - PubMed
-
- Boggio PS, et al. Effects of transcranial direct current stimulation on working memory in patients with Parkinson’s disease. J Neurol Sci. 2006;249(1):31–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

