Growth of hydroxyapatite coatings on biodegradable polymer microspheres
- PMID: 20161578
- PMCID: PMC2806690
- DOI: 10.1021/am9001716
Growth of hydroxyapatite coatings on biodegradable polymer microspheres
Abstract
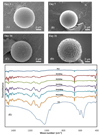
Mineral-coated microspheres were prepared via a bioinspired, heterogeneous nucleation process at physiological temperature. Poly(d,l-lactide-co-glycolide) (PLG) microspheres were fabricated via a water-in-oil-in-water emulsion method and were mineral-coated via incubation in a modified simulated body fluid (mSBF). X-ray diffraction, Fourier transform infrared spectroscopy, and scanning electron microscopy with associated energy-dispersive X-ray spectroscopy confirmed the presence of a continuous mineral coating on the microspheres. The mineral grown on the PLG microsphere surface has characteristics analogous to those of bone mineral (termed "bonelike" mineral), with a carbonate-containing hydroxyapatite phase and a porous structure of platelike crystals at the nanometer scale. The assembly of mineral-coated microspheres into aggregates was observed when microsphere concentrations above 0.50 mg/mL were incubated in mSBF for 7 days, and the size of the aggregates was dependent on the microsphere concentration in solution. In vitro mineral dissolution studies performed in Tris-buffered saline confirmed that the mineral formed was resorbable. A surfactant additive (Tween 20) was incorporated into mSBF to gain insight into the mineral growth process, and Tween 20 not only prevented aggregation but also significantly inhibited mineral formation and influenced the characteristics of the mineral formed on the surface of PLG microspheres. Taken together, these findings indicate that mineral-coated PLG microspheres or mineral-coated microsphere aggregates can be synthesized in a controllable manner using a bioinspired process. These materials may be useful in a range of applications, including controlled drug delivery and biomolecule purification.
Keywords: HAP chromatography; PLG microsphere; bioinspired; drug delivery; mineralization.
Figures







Similar articles
-
Bioinspired growth of crystalline carbonate apatite on biodegradable polymer substrata.J Am Chem Soc. 2002 Mar 6;124(9):1910-7. doi: 10.1021/ja012433n. J Am Chem Soc. 2002. PMID: 11866603
-
In vitro and in vivo evaluations of nanocrystalline Zn-doped carbonated hydroxyapatite/alginate microspheres: zinc and calcium bioavailability and bone regeneration.Int J Nanomedicine. 2019 May 10;14:3471-3490. doi: 10.2147/IJN.S197157. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31190805 Free PMC article.
-
Growth of continuous bonelike mineral within porous poly(lactide-co-glycolide) scaffolds in vitro.J Biomed Mater Res. 2000 Apr;50(1):50-8. doi: 10.1002/(sici)1097-4636(200004)50:1<50::aid-jbm8>3.0.co;2-f. J Biomed Mater Res. 2000. PMID: 10644963
-
Biodegradable recombinant human erythropoietin loaded microspheres prepared from linear and star-branched block copolymers: influence of encapsulation technique and polymer composition on particle characteristics.J Control Release. 1999 Jun 2;59(3):309-25. doi: 10.1016/s0168-3659(99)00008-5. J Control Release. 1999. PMID: 10332063
-
Facile fabrication of poly(L-lactic acid) microsphere-incorporated calcium alginate/hydroxyapatite porous scaffolds based on Pickering emulsion templates.Colloids Surf B Biointerfaces. 2016 Apr 1;140:382-391. doi: 10.1016/j.colsurfb.2016.01.005. Epub 2016 Jan 6. Colloids Surf B Biointerfaces. 2016. PMID: 26774574
Cited by
-
A controlled release of antibiotics from calcium phosphate-coated poly(lactic-co-glycolic acid) particles and their in vitro efficacy against Staphylococcus aureus biofilm.J Mater Sci Mater Med. 2014 Mar;25(3):747-57. doi: 10.1007/s10856-013-5125-9. Epub 2013 Dec 27. J Mater Sci Mater Med. 2014. PMID: 24370968
-
Multilayered Inorganic Microparticles for Tunable Dual Growth Factor Delivery.Adv Funct Mater. 2014 May 28;24(20):3082-3093. doi: 10.1002/adfm.201302859. Adv Funct Mater. 2014. PMID: 25342948 Free PMC article.
-
Tracking injectable microspheres in dynamic tissues with encapsulated superparamagnetic iron oxide nanoparticles.Macromol Biosci. 2012 Dec;12(12):1615-21. doi: 10.1002/mabi.201100489. Epub 2012 Nov 1. Macromol Biosci. 2012. PMID: 23124987 Free PMC article.
-
Multimodal Label-Free Imaging for Detecting Maturation of Engineered Osteogenic Grafts.ACS Biomater Sci Eng. 2019 Apr 8;5(4):1956-1966. doi: 10.1021/acsbiomaterials.9b00007. Epub 2019 Mar 27. ACS Biomater Sci Eng. 2019. PMID: 33405522 Free PMC article.
-
3-D Scaffold Platform for Optimized Non-viral Transfection of Multipotent Stem Cells.J Mater Chem B. 2014 Dec 14;2(46):8186-8193. doi: 10.1039/C4TB00957F. J Mater Chem B. 2014. PMID: 25541592 Free PMC article.
References
-
- Ducheyne P, Qui Q-Q. Biomaterials. 1999;20:2287–2303. - PubMed
-
- Habibovic P, Sees TM, van den Doel MA, van Blitterswijk CA, de Groot K. J. Biomed. Mater. Res., Part A. 2006;77A:747–762. - PubMed
-
- Colman A, Byers MJ, Primrose SB, Lyons A. Eur. J. Biochem. 1978;91:303–310. - PubMed
-
- Schroder E, Jonsson T, Poole L. Anal. Biochem. 2003;313:176–178. - PubMed
-
- Alt V, Pfefferle HJ, Kreuter J, Stahl JP, Pavlidis T, Meyer C, Mockwitz J, Wenisch S, Schnettler R. J. Control Release. 2004;99:103–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

