Mechanisms of brain iron transport: insight into neurodegeneration and CNS disorders
- PMID: 20161623
- PMCID: PMC2812924
- DOI: 10.4155/fmc.09.140
Mechanisms of brain iron transport: insight into neurodegeneration and CNS disorders
Abstract
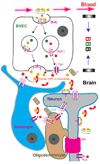
Trace metals such as iron, copper, zinc, manganese, and cobalt are essential cofactors for many cellular enzymes. Extensive research on iron, the most abundant transition metal in biology, has contributed to an increased understanding of the molecular machinery involved in maintaining its homeostasis in mammalian peripheral tissues. However, the cellular and intercellular iron transport mechanisms in the central nervous system (CNS) are still poorly understood. Accumulating evidence suggests that impaired iron metabolism is an initial cause of neurodegeneration, and several common genetic and sporadic neurodegenerative disorders have been proposed to be associated with dysregulated CNS iron homeostasis. This review aims to provide a summary of the molecular mechanisms of brain iron transport. Our discussion is focused on iron transport across endothelial cells of the blood-brain barrier and within the neuro- and glial-vascular units of the brain, with the aim of revealing novel therapeutic targets for neurodegenerative and CNS disorders.
Keywords: Blood-brain barrier (BBB); Reactive-Oxygen Species (ROS); brain vascular endothelial cell (BVEC); divalent metal transporter-1 (DMT1, Slc11a2); early endosome (EE); ferritin (Ft); ferroportin (Fpn); non-transferrin-bound iron (NTBI); transferrin (Tf); transient receptor potential mucolipin 1 (TRPML1).
Figures

Similar articles
-
Regulation of brain iron and copper homeostasis by brain barrier systems: implication in neurodegenerative diseases.Pharmacol Ther. 2012 Feb;133(2):177-88. doi: 10.1016/j.pharmthera.2011.10.006. Epub 2011 Nov 13. Pharmacol Ther. 2012. PMID: 22115751 Free PMC article. Review.
-
Brain iron homeostasis.Dan Med Bull. 2002 Nov;49(4):279-301. Dan Med Bull. 2002. PMID: 12553165 Review.
-
Endothelial cells are critical regulators of iron transport in a model of the human blood-brain barrier.J Cereb Blood Flow Metab. 2019 Nov;39(11):2117-2131. doi: 10.1177/0271678X18783372. Epub 2018 Jun 18. J Cereb Blood Flow Metab. 2019. PMID: 29911470 Free PMC article.
-
The role of transferrins and iron-related proteins in brain iron transport: applications to neurological diseases.Adv Protein Chem Struct Biol. 2021;123:133-162. doi: 10.1016/bs.apcsb.2020.09.002. Epub 2020 Dec 19. Adv Protein Chem Struct Biol. 2021. PMID: 33485481 Review.
-
Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat.J Neurosci Res. 2001 Dec 15;66(6):1198-207. doi: 10.1002/jnr.1256. J Neurosci Res. 2001. PMID: 11746453
Cited by
-
Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: implications for neuroprotection.Mol Neurobiol. 2014 Feb;49(1):222-33. doi: 10.1007/s12035-013-8514-7. Epub 2013 Jul 28. Mol Neurobiol. 2014. PMID: 23893294
-
The association of myelination in the internal capsule with iron deposition in the basal ganglia in macaques: a magnetic resonance imaging study.Quant Imaging Med Surg. 2020 Jul;10(7):1526-1539. doi: 10.21037/qims-19-1014. Quant Imaging Med Surg. 2020. PMID: 32676370 Free PMC article.
-
Targeting Iron Dyshomeostasis for Treatment of Neurodegenerative Disorders.CNS Drugs. 2019 Nov;33(11):1073-1086. doi: 10.1007/s40263-019-00668-6. CNS Drugs. 2019. PMID: 31556017 Free PMC article. Review.
-
Magnetic resonance evidence of increased iron content in subcortical brain regions in asymptomatic Alzheimer's disease.Hum Brain Mapp. 2023 Jun 1;44(8):3072-3083. doi: 10.1002/hbm.26263. Epub 2023 Mar 16. Hum Brain Mapp. 2023. PMID: 36929676 Free PMC article.
-
Association of Endogenous Erythropoietin Levels and Iron Status With Cognitive Functioning in the General Population.Front Aging Neurosci. 2022 Apr 8;14:862856. doi: 10.3389/fnagi.2022.862856. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35462689 Free PMC article.
References
-
- Fillebeen C, et al. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J Biol Chem. 1999;274(11):7011–7017. - PubMed
-
- Richardson DR, Ponka P. The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim Biophys Acta. 1997;1331(1):1–40. - PubMed
-
- Rothenberger S, et al. Coincident expression and distribution of melanotransferrin and transferrin receptor in human brain capillary endothelium. Brain Res. 1996;712(1):117–121. - PubMed
-
- Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117(3):285–297. - PubMed
-
- Moos T, et al. Iron trafficking inside the brain. J Neurochem. 2007;103(5):1730–1740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
