Natural Products as a Foundation for Drug Discovery
- PMID: 20161632
- PMCID: PMC2813068
- DOI: 10.1002/0471141755.ph0911s46
Natural Products as a Foundation for Drug Discovery
Abstract
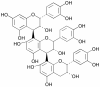
Natural products have contributed to the development of many drugs for diverse indications. While most U.S. pharmaceutical companies have reduced or eliminated their in-house natural product groups, new paradigms and new enterprises have evolved to carry on a role for natural products in the pharmaceutical industry. Many of the reasons for the decline in popularity of natural products are being addressed by the development of new techniques for screening and production. This overview aims to inform pharmacologists of current strategies and techniques that make natural products a viable strategic choice for inclusion in drug discovery programs.
Figures

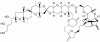





References
-
- Aicher TD, Buszek KR, Fang FG, Forsyth CJ, Jung SH, Kishi Y, Matelich MC, Scola PM, Spero DM, Yoon SK. Total synthesis of halichondrin B and norhalichondrin B. J.Am.Chem.Soc. 1992;114:3162–3164.
-
- Akaike N, Hattori K, Oomura Y, Carpenter DO. Bicuculline and picrotoxin Block γ-aminobutyric acid-gated Cl− conductance by different mechanisms. Experientia. 1985;41:70–71. - PubMed
-
- Arneric SP, Holladay M, Williams M. Neuronal nicotinic receptors: a perspective on two decades of drug discovery research. Biochem.Pharmacol. 2007;74:1092–1101. - PubMed
-
- Badio B, Daly JW. Epibatidine, a potent analgesic and nicotinic agonist. Mol.Pharmacol. 1994;45:563–569. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

