Defective glycinergic synaptic transmission in zebrafish motility mutants
- PMID: 20161699
- PMCID: PMC2813725
- DOI: 10.3389/neuro.02.026.2009
Defective glycinergic synaptic transmission in zebrafish motility mutants
Abstract
Glycine is a major inhibitory neurotransmitter in the spinal cord and brainstem. Recently, in vivo analysis of glycinergic synaptic transmission has been pursued in zebrafish using molecular genetics. An ENU mutagenesis screen identified two behavioral mutants that are defective in glycinergic synaptic transmission. Zebrafish bandoneon (beo) mutants have a defect in glrbb, one of the duplicated glycine receptor (GlyR) beta subunit genes. These mutants exhibit a loss of glycinergic synaptic transmission due to a lack of synaptic aggregation of GlyRs. Due to the consequent loss of reciprocal inhibition of motor circuits between the two sides of the spinal cord, motor neurons activate simultaneously on both sides resulting in bilateral contraction of axial muscles of beo mutants, eliciting the so-called 'accordion' phenotype. Similar defects in GlyR subunit genes have been observed in several mammals and are the basis for human hyperekplexia/startle disease. By contrast, zebrafish shocked (sho) mutants have a defect in slc6a9, encoding GlyT1, a glycine transporter that is expressed by astroglial cells surrounding the glycinergic synapse in the hindbrain and spinal cord. GlyT1 mediates rapid uptake of glycine from the synaptic cleft, terminating synaptic transmission. In zebrafish sho mutants, there appears to be elevated extracellular glycine resulting in persistent inhibition of postsynaptic neurons and subsequent reduced motility, causing the 'twitch-once' phenotype. We review current knowledge regarding zebrafish 'accordion' and 'twitch-once' mutants, including beo and sho, and report the identification of a new alpha2 subunit that revises the phylogeny of zebrafish GlyRs.
Keywords: behavior; glycine; motility; receptor; synapse; transporter; zebrafish.
Figures

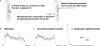


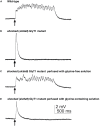
Similar articles
-
Defects of the Glycinergic Synapse in Zebrafish.Front Mol Neurosci. 2016 Jun 29;9:50. doi: 10.3389/fnmol.2016.00050. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27445686 Free PMC article. Review.
-
Zebrafish bandoneon mutants display behavioral defects due to a mutation in the glycine receptor beta-subunit.Proc Natl Acad Sci U S A. 2005 Jun 7;102(23):8345-50. doi: 10.1073/pnas.0500862102. Epub 2005 May 31. Proc Natl Acad Sci U S A. 2005. PMID: 15928085 Free PMC article.
-
Distinct phenotypes in zebrafish models of human startle disease.Neurobiol Dis. 2013 Dec;60:139-51. doi: 10.1016/j.nbd.2013.09.002. Epub 2013 Sep 9. Neurobiol Dis. 2013. PMID: 24029548 Free PMC article.
-
The startle disease mutation α1S270T predicts shortening of glycinergic synaptic currents.J Physiol. 2020 Aug;598(16):3417-3438. doi: 10.1113/JP279803. Epub 2020 Jun 18. J Physiol. 2020. PMID: 32445491 Free PMC article.
-
The biological role of the glycinergic synapse in early zebrafish motility.Neurosci Res. 2011 Sep;71(1):1-11. doi: 10.1016/j.neures.2011.06.003. Epub 2011 Jun 17. Neurosci Res. 2011. PMID: 21712054 Review.
Cited by
-
Defects of the Glycinergic Synapse in Zebrafish.Front Mol Neurosci. 2016 Jun 29;9:50. doi: 10.3389/fnmol.2016.00050. eCollection 2016. Front Mol Neurosci. 2016. PMID: 27445686 Free PMC article. Review.
-
Frontiers in molecular neuroscience - résumé and perspective.Front Mol Neurosci. 2011 Dec 30;4:58. doi: 10.3389/fnmol.2011.00058. eCollection 2011. Front Mol Neurosci. 2011. PMID: 22232574 Free PMC article. No abstract available.
-
Behavioral genetics in larval zebrafish: learning from the young.Dev Neurobiol. 2012 Mar;72(3):366-72. doi: 10.1002/dneu.20872. Dev Neurobiol. 2012. PMID: 22328273 Free PMC article. Review.
-
Mitochondria in Embryogenesis: An Organellogenesis Perspective.Front Cell Dev Biol. 2019 Nov 22;7:282. doi: 10.3389/fcell.2019.00282. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31824944 Free PMC article.
-
Flux coupling, not specificity, shapes the transport and phylogeny of SLC6 glycine transporters.Proc Natl Acad Sci U S A. 2022 Oct 11;119(41):e2205874119. doi: 10.1073/pnas.2205874119. Epub 2022 Oct 3. Proc Natl Acad Sci U S A. 2022. PMID: 36191186 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

