A novel high-affinity sucrose transporter is required for virulence of the plant pathogen Ustilago maydis
- PMID: 20161717
- PMCID: PMC2817709
- DOI: 10.1371/journal.pbio.1000303
A novel high-affinity sucrose transporter is required for virulence of the plant pathogen Ustilago maydis
Abstract
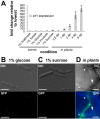
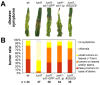
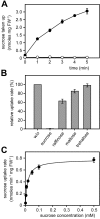
Plant pathogenic fungi cause massive yield losses and affect both quality and safety of food and feed produced from infected plants. The main objective of plant pathogenic fungi is to get access to the organic carbon sources of their carbon-autotrophic hosts. However, the chemical nature of the carbon source(s) and the mode of uptake are largely unknown. Here, we present a novel, plasma membrane-localized sucrose transporter (Srt1) from the corn smut fungus Ustilago maydis and its characterization as a fungal virulence factor. Srt1 has an unusually high substrate affinity, is absolutely sucrose specific, and allows the direct utilization of sucrose at the plant/fungal interface without extracellular hydrolysis and, thus, without the production of extracellular monosaccharides known to elicit plant immune responses. srt1 is expressed exclusively during infection, and its deletion strongly reduces fungal virulence. This emphasizes the central role of this protein both for efficient carbon supply and for avoidance of apoplastic signals potentially recognized by the host.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

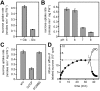
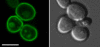

Comment in
-
Living the sweet life: how does a plant pathogenic fungus acquire sugar from plants?PLoS Biol. 2010 Feb 9;8(2):e1000308. doi: 10.1371/journal.pbio.1000308. PLoS Biol. 2010. PMID: 20161721 Free PMC article. No abstract available.
Similar articles
-
Living the sweet life: how does a plant pathogenic fungus acquire sugar from plants?PLoS Biol. 2010 Feb 9;8(2):e1000308. doi: 10.1371/journal.pbio.1000308. PLoS Biol. 2010. PMID: 20161721 Free PMC article. No abstract available.
-
Inverse pH regulation of plant and fungal sucrose transporters: a mechanism to regulate competition for sucrose at the host/pathogen interface?PLoS One. 2010 Aug 26;5(8):e12429. doi: 10.1371/journal.pone.0012429. PLoS One. 2010. PMID: 20865151 Free PMC article.
-
The core effector Cce1 is required for early infection of maize by Ustilago maydis.Mol Plant Pathol. 2018 Oct;19(10):2277-2287. doi: 10.1111/mpp.12698. Epub 2018 Aug 16. Mol Plant Pathol. 2018. PMID: 29745456 Free PMC article.
-
Protein glycosylation in the phytopathogen Ustilago maydis: From core oligosaccharide synthesis to the ER glycoprotein quality control system, a genomic analysis.Fungal Genet Biol. 2010 Sep;47(9):727-35. doi: 10.1016/j.fgb.2010.06.004. Epub 2010 Jun 8. Fungal Genet Biol. 2010. PMID: 20554055 Review.
-
Ustilago maydis effectors and their impact on virulence.Nat Rev Microbiol. 2017 Jul;15(7):409-421. doi: 10.1038/nrmicro.2017.33. Epub 2017 May 8. Nat Rev Microbiol. 2017. PMID: 28479603 Review.
Cited by
-
Identification and Characterization of Two Transmembrane Proteins Required for Virulence of Ustilago maydis.Front Plant Sci. 2021 May 21;12:669835. doi: 10.3389/fpls.2021.669835. eCollection 2021. Front Plant Sci. 2021. PMID: 34093627 Free PMC article.
-
Ustilago maydis: dissecting the molecular interface between pathogen and plant.PLoS Pathog. 2012;8(11):e1002955. doi: 10.1371/journal.ppat.1002955. Epub 2012 Nov 1. PLoS Pathog. 2012. PMID: 23133380 Free PMC article. Review. No abstract available.
-
Living the sweet life: how does a plant pathogenic fungus acquire sugar from plants?PLoS Biol. 2010 Feb 9;8(2):e1000308. doi: 10.1371/journal.pbio.1000308. PLoS Biol. 2010. PMID: 20161721 Free PMC article. No abstract available.
-
The transcription factor Rbf1 is the master regulator for b-mating type controlled pathogenic development in Ustilago maydis.PLoS Pathog. 2010 Aug 5;6(8):e1001035. doi: 10.1371/journal.ppat.1001035. PLoS Pathog. 2010. PMID: 20700446 Free PMC article.
-
Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis.Nat Commun. 2010 Jul 27;1:48. doi: 10.1038/ncomms1046. Nat Commun. 2010. PMID: 20975705 Review.
References
-
- Glazebrook J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Rev Phytopathol. 2005;43:205–227. - PubMed
-
- Kliebenstein D. J, Rowe H. C. Ecological costs of biotrophic versus necrotrophic pathogen resistance, the hypersensitive response and signal transduction. Plant Sci. 2008;174:551–556.
-
- Martinez-Espinoza A. D, Garcia-Pedrajas M. D, Gold S. E. The Ustilaginales as plant pest and model systems. Fungal Genet Biol. 2002;35:1–20. - PubMed
-
- Mendgen K, Hahn M. Plant infection and the establishment of fungal biotrophy. Trends Plant Sci. 2002;7:352–356. - PubMed
-
- Bauer R, Oberwinkler F, Vánky K. Ultrastructural markers and systematics in smut fungi and allied taxa. Can J Bot. 1997;75:1273–1314.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

