Schistosoma mansoni Stomatin like protein-2 is located in the tegument and induces partial protection against challenge infection
- PMID: 20161725
- PMCID: PMC2817717
- DOI: 10.1371/journal.pntd.0000597
Schistosoma mansoni Stomatin like protein-2 is located in the tegument and induces partial protection against challenge infection
Abstract
Background: Schistosomiasis affects more than 200 million individuals worldwide, with a further 650 million living at risk of infection, constituting a severe health problem in developing countries. Even though an effective treatment exists, it does not prevent re-infection, and the development of an effective vaccine still remains the most desirable means of control for this disease.
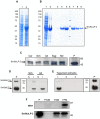
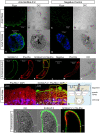
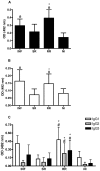
Methodology/principal findings: Herein, we report the cloning and characterization of a S. mansoni Stomatin-like protein 2 (SmStoLP-2). In silico analysis predicts three putative sites for palmitoylation (Cys11, Cys61 and Cys330), which could contribute to protein membrane association; and a putative mitochondrial targeting sequence, similar to that described for human Stomatin-like protein 2 (HuSLP-2). The protein was detected by Western blot with comparable levels in all stages across the parasite life cycle. Fractionation by differential centrifugation of schistosome tegument suggested that SmStoLP-2 displays a dual targeting to the tegument membranes and mitochondria; additionally, immunolocalization experiments confirm its localization in the tegument of the adult worms and, more importantly, in 7-day-old schistosomula. Analysis of the antibody isotype profile to rSmStoLP-2 in the sera of patients living in endemic areas for schistosomiasis revealed that IgG1, IgG2, IgG3 and IgA antibodies were predominant in sera of individuals resistant to reinfection as compared to those susceptible. Next, immunization of mice with rSmStoLP-2 engendered a 30%-32% reduction in adult worm burden. Protective immunity in mice was associated with specific anti-rSmStoLP-2 IgG1 and IgG2a antibodies and elevated production of IFN-gamma and TNF-alpha, while no IL-4 production was detected, suggesting a Th1-predominant immune response.
Conclusions/significance: Data presented here demonstrate that SmStoLP-2 is a novel tegument protein located in the host-parasite interface. It is recognized by different subclasses of antibodies in patients resistant and susceptible to reinfection and, based on the data from murine studies, shows protective potential against schistosomiasis. These results indicate that SmStoLP-2 could be useful in a combination vaccine.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

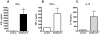

Similar articles
-
Vaccination with enzymatically cleaved GPI-anchored proteins from Schistosoma mansoni induces protection against challenge infection.Clin Dev Immunol. 2012;2012:962538. doi: 10.1155/2012/962538. Epub 2012 Aug 15. Clin Dev Immunol. 2012. PMID: 22927873 Free PMC article.
-
Schistosoma mansoni tegument protein Sm29 is able to induce a Th1-type of immune response and protection against parasite infection.PLoS Negl Trop Dis. 2008 Oct 1;2(10):e308. doi: 10.1371/journal.pntd.0000308. PLoS Negl Trop Dis. 2008. PMID: 18827884 Free PMC article.
-
Immunization with SmIg, a novel tegument protein from Schistosoma mansoni, fails to induce protection in mice but reduces liver pathology.Parasitology. 2010 Jun;137(7):1079-88. doi: 10.1017/S0031182009991387. Epub 2009 Oct 16. Parasitology. 2010. PMID: 19835649
-
Human schistosomiasis mansoni: immune responses during acute and chronic phases of the infection.Acta Trop. 2008 Nov-Dec;108(2-3):109-17. doi: 10.1016/j.actatropica.2008.05.027. Epub 2008 Jun 5. Acta Trop. 2008. PMID: 18577364 Review.
-
Pre-clinical studies of Schistosoma mansoni vaccines: A scoping review.PLoS Negl Trop Dis. 2025 Jun 2;19(6):e0012956. doi: 10.1371/journal.pntd.0012956. eCollection 2025 Jun. PLoS Negl Trop Dis. 2025. PMID: 40455825 Free PMC article.
Cited by
-
Vaccination with enzymatically cleaved GPI-anchored proteins from Schistosoma mansoni induces protection against challenge infection.Clin Dev Immunol. 2012;2012:962538. doi: 10.1155/2012/962538. Epub 2012 Aug 15. Clin Dev Immunol. 2012. PMID: 22927873 Free PMC article.
-
Exploring the immune interactions between Oncomelania hupensis and Schistosoma japonicum, with a cross-comparison of immunological research progress in other intermediate host snails.Parasit Vectors. 2023 Dec 13;16(1):453. doi: 10.1186/s13071-023-06011-9. Parasit Vectors. 2023. PMID: 38093363 Free PMC article. Review.
-
Sm10.3, a member of the micro-exon gene 4 (MEG-4) family, induces erythrocyte agglutination in vitro and partially protects vaccinated mice against Schistosoma mansoni infection.PLoS Negl Trop Dis. 2014 Mar 20;8(3):e2750. doi: 10.1371/journal.pntd.0002750. eCollection 2014 Mar. PLoS Negl Trop Dis. 2014. PMID: 24651069 Free PMC article.
-
Schistosome syntenin partially protects vaccinated mice against Schistosoma mansoni infection.PLoS Negl Trop Dis. 2014 Aug 21;8(8):e3107. doi: 10.1371/journal.pntd.0003107. eCollection 2014 Aug. PLoS Negl Trop Dis. 2014. PMID: 25144756 Free PMC article.
-
Schistosoma mansoni SmKI-1 or Its C-Terminal Fragment Induces Partial Protection Against S. mansoni Infection in Mice.Front Immunol. 2018 Jul 30;9:1762. doi: 10.3389/fimmu.2018.01762. eCollection 2018. Front Immunol. 2018. PMID: 30105029 Free PMC article.
References
-
- WHO. TDR Strategic Direction for Research: Schistosomiasis. Geneve: World Health Organization; 2002.
-
- Harder A. Chemotherapeutic approaches to schistosomes: current knowledge and outlook. Parasitol Res. 2002;88:395–397. - PubMed
-
- Bergquist NR. Schistosomiasis vaccine development: approaches and prospects. Mem Inst Oswaldo Cruz. 1995;90:221–227. - PubMed
-
- Ismail M, Botros S, Metwally A, William S, Farghally A, et al. Resistance to praziquantel: direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg. 1999;60:932–935. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

