PLUNC is a novel airway surfactant protein with anti-biofilm activity
- PMID: 20161732
- PMCID: PMC2817724
- DOI: 10.1371/journal.pone.0009098
PLUNC is a novel airway surfactant protein with anti-biofilm activity
Abstract
Background: The PLUNC ("Palate, lung, nasal epithelium clone") protein is an abundant secretory product of epithelia present throughout the conducting airways of humans and other mammals, which is evolutionarily related to the lipid transfer/lipopolysaccharide binding protein (LT/LBP) family. Two members of this family--the bactericidal/permeability increasing protein (BPI) and the lipopolysaccharide binding protein (LBP)--are innate immune molecules with recognized roles in sensing and responding to Gram negative bacteria, leading many to propose that PLUNC may play a host defense role in the human airways.
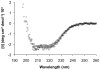
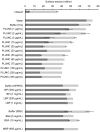
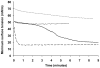
Methodology/principal findings: Based on its marked hydrophobicity, we hypothesized that PLUNC may be an airway surfactant. We found that purified recombinant human PLUNC greatly enhanced the ability of aqueous solutions to spread on a hydrophobic surface. Furthermore, we discovered that PLUNC significantly reduced surface tension at the air-liquid interface in aqueous solutions, indicating novel and biologically relevant surfactant properties. Of note, surface tensions achieved by adding PLUNC to solutions are very similar to measurements of the surface tension in tracheobronchial secretions from humans and animal models. Because surfactants of microbial origin can disperse matrix-encased bacterial clusters known as biofilms [1], we hypothesized that PLUNC may also have anti-biofilm activity. We found that, at a physiologically relevant concentration, PLUNC inhibited biofilm formation by the airway pathogen Pseudomonas aeruginosa in an in vitro model.
Conclusions/significance: Our data suggest that the PLUNC protein contributes to the surfactant properties of airway secretions, and that this activity may interfere with biofilm formation by an airway pathogen.
Conflict of interest statement
Figures

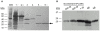

Similar articles
-
PLUNC: a multifunctional surfactant of the airways.Biochem Soc Trans. 2011 Aug;39(4):1012-6. doi: 10.1042/BST0391012. Biochem Soc Trans. 2011. PMID: 21787339 Free PMC article.
-
SPLUNC1/BPIFA1 contributes to pulmonary host defense against Klebsiella pneumoniae respiratory infection.Am J Pathol. 2013 May;182(5):1519-31. doi: 10.1016/j.ajpath.2013.01.050. Epub 2013 Mar 15. Am J Pathol. 2013. PMID: 23499554 Free PMC article.
-
Purification and characterization of PLUNC from human tracheobronchial secretions.Am J Respir Cell Mol Biol. 2004 Feb;30(2):184-92. doi: 10.1165/rcmb.2003-0142OC. Epub 2003 Aug 14. Am J Respir Cell Mol Biol. 2004. PMID: 12920053
-
Comparative analysis of the PLUNC (palate, lung and nasal epithelium clone) protein families.Biochem Soc Trans. 2003 Aug;31(Pt 4):806-9. doi: 10.1042/bst0310806. Biochem Soc Trans. 2003. PMID: 12887310 Review.
-
The Multifunctional Roles of Short Palate, Lung, and Nasal Epithelium Clone 1 in Regulating Airway Surface Liquid and Participating in Airway Host Defense.J Interferon Cytokine Res. 2021 Apr;41(4):139-148. doi: 10.1089/jir.2020.0141. J Interferon Cytokine Res. 2021. PMID: 33885339 Review.
Cited by
-
Evaluation of correlation of cell cycle proteins and Ki-67 interaction in paranasal sinus inverted papilloma prognosis and squamous cell carcinoma transformation.Biomed Res Int. 2014;2014:634945. doi: 10.1155/2014/634945. Epub 2014 Jun 12. Biomed Res Int. 2014. PMID: 25013792 Free PMC article.
-
Interleukin-13 Inhibits Lipopolysaccharide-Induced BPIFA1 Expression in Nasal Epithelial Cells.PLoS One. 2015 Dec 8;10(12):e0143484. doi: 10.1371/journal.pone.0143484. eCollection 2015. PLoS One. 2015. PMID: 26646664 Free PMC article.
-
The Acari Hypothesis, V: deciphering allergenicity.Front Allergy. 2024 Nov 1;5:1454292. doi: 10.3389/falgy.2024.1454292. eCollection 2024. Front Allergy. 2024. PMID: 39552700 Free PMC article.
-
Surfactant and its role in the pathobiology of pulmonary infection.Microbes Infect. 2012 Jan;14(1):17-25. doi: 10.1016/j.micinf.2011.08.019. Epub 2011 Sep 10. Microbes Infect. 2012. PMID: 21945366 Free PMC article. Review.
-
Molecular biology of BPIFB1 and its advances in disease.Ann Transl Med. 2020 May;8(10):651. doi: 10.21037/atm-20-3462. Ann Transl Med. 2020. PMID: 32566588 Free PMC article. Review.
References
-
- Boles BR, Thoendel M, Singh PK. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol Microbiol. 2005;57:1210–1223. - PubMed
-
- Gil J, Weibel ER. Extracellular lining of bronchioles after perfusion-fixation of rat lungs for electron microscopy. Anat Rec. 1971;169:185–199. - PubMed
-
- Serrano AG, Perez-Gil J. Protein-lipid interactions and surface activity in the pulmonary surfactant system. Chem Phys Lipids. 2006;141:105–118. - PubMed
-
- Whitsett JA, Weaver TE. Hydrophobic surfactant proteins in lung function and disease. N Engl J Med. 2002;347:2141–2148. - PubMed
-
- Bernhard W, Haagsman HP, Tschernig T, Poets CF, Postle AD, et al. Conductive airway surfactant: surface-tension function, biochemical composition, and possible alveolar origin. Am J Respir Cell Mol Biol. 1997;17:41–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

