Causal relationship of susceptibility genes to ischemic stroke: comparison to ischemic heart disease and biochemical determinants
- PMID: 20161734
- PMCID: PMC2817726
- DOI: 10.1371/journal.pone.0009136
Causal relationship of susceptibility genes to ischemic stroke: comparison to ischemic heart disease and biochemical determinants
Abstract
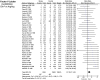
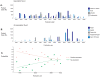
Interrelationships between genetic and biochemical factors underlying ischemic stroke and ischemic heart disease are poorly understood. We: 1) undertook the most comprehensive meta-analysis of genetic polymorphisms in ischemic stroke to date; 2) compared genetic determinants of ischemic stroke with those of ischemic heart disease, and 3) compared effect sizes of gene-stroke associations with those predicted from independent biochemical data using a mendelian randomization strategy. Electronic databases were searched up to January 2009. We identified: 1) 187 ischemic stroke studies (37,481 cases; 95,322 controls) interrogating 43 polymorphisms in 29 genes; 2) 13 meta-analyses testing equivalent polymorphisms in ischemic heart disease; and 3) for the top five gene-stroke associations, 146 studies (65,703 subjects) describing equivalent gene-biochemical relationships, and 28 studies (46,928 subjects) describing biochemical-stroke relationships. Meta-analyses demonstrated positive associations with ischemic stroke for factor V Leiden Gln506, ACE I/D, MTHFR C677T, prothrombin G20210A, PAI-1 5G allele and glycoprotein IIIa Leu33Pro polymorphisms (ORs: 1.11 - 1.60). Most genetic associations show congruent levels of risk comparing ischemic stroke with ischemic heart disease, but three genes--glycoprotein IIIa, PAI-1 and angiotensinogen--show significant dissociations. The magnitudes of stroke risk observed for factor V Leiden, ACE, MTHFR and prothrombin, but not PAI-1, polymorphisms, are consistent with risks associated with equivalent changes in activated protein C resistance, ACE activity, homocysteine, prothrombin, and PAI-1 levels, respectively. Our results demonstrate causal relationships for four of the most robust genes associated with stroke while also showing that PAI-1 4G/5G polymorphism influences cardiovascular risk via a mechanism not simply related to plasma levels of PAI-1 (or tPA) alone.
Conflict of interest statement
Figures



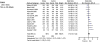
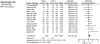








References
-
- Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol. 2009;8:345–354. - PubMed
-
- Arnett DK, Baird AE, Barkley RA, Basson CT, Boerwinkle E, et al. American Heart Association Council on Epidemiology and Prevention; American Heart Association Stroke Council; Functional Genomics and Translational Biology Interdisciplinary Working Group; Relevance of genetics and genomics for prevention and treatment of cardiovascular disease. Circulation. 2007;115:2878–901. - PubMed
-
- Ioannidis JP, Boffetta P, Little J, O'Brien TR, Uitterlinden AG, et al. Assessment of cumulative evidence on genetic associations: interim guidelines. Int J Epidemiol. 2008;37:120–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

