Phenylbutyric acid rescues endoplasmic reticulum stress-induced suppression of APP proteolysis and prevents apoptosis in neuronal cells
- PMID: 20161760
- PMCID: PMC2817752
- DOI: 10.1371/journal.pone.0009135
Phenylbutyric acid rescues endoplasmic reticulum stress-induced suppression of APP proteolysis and prevents apoptosis in neuronal cells
Abstract
Background: The familial and sporadic forms of Alzheimer's disease (AD) have an identical pathology with a severe disparity in the time of onset [1]. The pathological similarity suggests that epigenetic processes may phenocopy the Familial Alzheimer's disease (FAD) mutations within sporadic AD. Numerous groups have demonstrated that FAD mutations in presenilin result in 'loss of function' of gamma-secretase mediated APP cleavage [2], [3], [4], [5]. Accordingly, ER stress is prominent within the pathologically impacted brain regions in AD patients [6] and is reported to inhibit APP trafficking through the secretory pathway [7], [8]. As the maturation of APP and the cleaving secretases requires trafficking through the secretory pathway [9], [10], [11], we hypothesized that ER stress may block trafficking requisite for normal levels of APP cleavage and that the small molecular chaperone 4-phenylbutyrate (PBA) may rescue the proteolytic deficit.
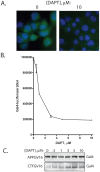
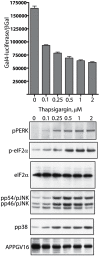
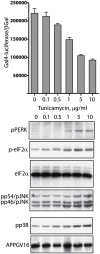
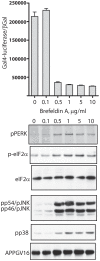
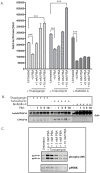
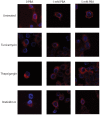
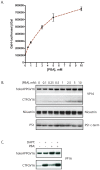
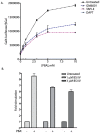
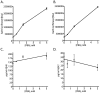
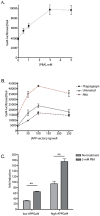
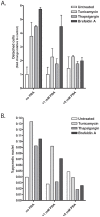
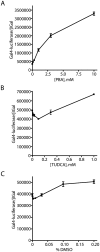
Methodology/principal findings: The APP-Gal4VP16/Gal4-reporter screen was stably incorporated into neuroblastoma cells in order to assay gamma-secretase mediated APP proteolysis under normal and pharmacologically induced ER stress conditions. Three unrelated pharmacological agents (tunicamycin, thapsigargin and brefeldin A) all repressed APP proteolysis in parallel with activation of unfolded protein response (UPR) signaling-a biochemical marker of ER stress. Co-treatment of the gamma-secretase reporter cells with PBA blocked the repressive effects of tunicamycin and thapsigargin upon APP proteolysis, UPR activation, and apoptosis. In unstressed cells, PBA stimulated gamma-secretase mediated cleavage of APP by 8-10 fold, in the absence of any significant effects upon amyloid production, by promoting APP trafficking through the secretory pathway and the stimulation of the non-pathogenic alpha/gamma-cleavage.
Conclusions/significance: ER stress represses gamma-secretase mediated APP proteolysis, which replicates some of the proteolytic deficits associated with the FAD mutations. The small molecular chaperone PBA can reverse ER stress induced effects upon APP proteolysis, trafficking and cellular viability. Pharmaceutical agents, such as PBA, that stimulate alpha/gamma-cleavage of APP by modifying intracellular trafficking should be explored as AD therapeutics.
Conflict of interest statement
Figures
Similar articles
-
Phenylbutyric acid reduces amyloid plaques and rescues cognitive behavior in AD transgenic mice.Aging Cell. 2011 Jun;10(3):418-28. doi: 10.1111/j.1474-9726.2011.00680.x. Epub 2011 Mar 22. Aging Cell. 2011. PMID: 21272191
-
Retention in endoplasmic reticulum 1 (RER1) modulates amyloid-β (Aβ) production by altering trafficking of γ-secretase and amyloid precursor protein (APP).J Biol Chem. 2012 Nov 23;287(48):40629-40. doi: 10.1074/jbc.M112.418442. Epub 2012 Oct 5. J Biol Chem. 2012. PMID: 23043097 Free PMC article.
-
Novel alpha-secretase cleavage of Alzheimer's amyloid beta precursor protein in the endoplasmic reticulum of COS7 cells.Neurosci Lett. 2005 Mar 7;376(1):14-9. doi: 10.1016/j.neulet.2004.11.032. Epub 2004 Dec 8. Neurosci Lett. 2005. PMID: 15694266
-
[Molecular pharmacological studies on the protection mechanism against endoplasmic reticulum stress-induced neurodegenerative disease].Yakugaku Zasshi. 2012;132(12):1437-42. doi: 10.1248/yakushi.12-00249. Yakugaku Zasshi. 2012. PMID: 23208051 Review. Japanese.
-
Trafficking and proteolytic processing of APP.Cold Spring Harb Perspect Med. 2012 May;2(5):a006270. doi: 10.1101/cshperspect.a006270. Cold Spring Harb Perspect Med. 2012. PMID: 22553493 Free PMC article. Review.
Cited by
-
Potential of chromatin modifying compounds for the treatment of Alzheimer's disease.Pathobiol Aging Age Relat Dis. 2012;2. doi: 10.3402/pba.v2i0.14980. Epub 2012 Feb 20. Pathobiol Aging Age Relat Dis. 2012. PMID: 22953035 Free PMC article.
-
Obesity induces hypothalamic endoplasmic reticulum stress and impairs proopiomelanocortin (POMC) post-translational processing.J Biol Chem. 2013 Jun 14;288(24):17675-88. doi: 10.1074/jbc.M113.475343. Epub 2013 May 2. J Biol Chem. 2013. PMID: 23640886 Free PMC article.
-
Potential for therapeutic manipulation of the UPR in disease.Semin Immunopathol. 2013 May;35(3):351-73. doi: 10.1007/s00281-013-0370-z. Epub 2013 Apr 10. Semin Immunopathol. 2013. PMID: 23572207 Free PMC article. Review.
-
Tauopathy induced by low level expression of a human brain-derived tau fragment in mice is rescued by phenylbutyrate.Brain. 2016 Aug;139(Pt 8):2290-306. doi: 10.1093/brain/aww137. Epub 2016 Jun 12. Brain. 2016. PMID: 27297240 Free PMC article.
-
The roles of oxidative stress, endoplasmic reticulum stress, and autophagy in aldosterone/mineralocorticoid receptor-induced podocyte injury.Lab Invest. 2015 Dec;95(12):1374-86. doi: 10.1038/labinvest.2015.118. Epub 2015 Sep 28. Lab Invest. 2015. PMID: 26414307
References
-
- Selkoe DJ, Podlisny MB. Deciphering the genetic basis of Alzheimer's disease. Annu Rev Genomics Hum Genet. 2002;3:67–99. - PubMed
-
- Kumar-Singh S, Theuns J, Van Broeck B, Pirici D, Vennekens K, et al. Mean age-of-onset of familial alzheimer disease caused by presenilin mutations correlates with both increased Abeta42 and decreased Abeta40. Hum Mutat. 2006;27:686–695. - PubMed
-
- Bentahir M, Nyabi O, Verhamme J, Tolia A, Horre K, et al. Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J Neurochem. 2006;96:732–742. - PubMed
-
- Wiley JC, Hudson M, Kanning KC, Schecterson LC, Bothwell M. Familial Alzheimer's disease mutations inhibit gamma-secretase-mediated liberation of beta-amyloid precursor protein carboxy-terminal fragment. J Neurochem. 2005;94:1189–1201. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

