Gab2 promotes hematopoietic stem cell maintenance and self-renewal synergistically with STAT5
- PMID: 20161778
- PMCID: PMC2818849
- DOI: 10.1371/journal.pone.0009152
Gab2 promotes hematopoietic stem cell maintenance and self-renewal synergistically with STAT5
Abstract
Background: Grb2-associated binding (Gab) adapter proteins play major roles in coordinating signaling downstream of hematopoietic cytokine receptors. In hematopoietic cells, Gab2 can modulate phosphatidylinositol-3 kinase and mitogen associated protein kinase activities and regulate the long-term multilineage competitive repopulating activity of hematopoietic stem cells (HSCs). Gab2 may also act in a linear pathway upstream or downstream of signal transducer and activator of transcription-5 (STAT5), a major positive regulator of HSC function. Therefore, we aimed to determine whether Gab2 and STAT5 function in hematopoiesis in a redundant or non-redundant manner.
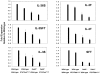
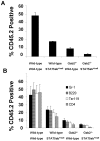
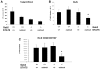
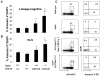
Methodology/principal findings: To do this we generated Gab2 mutant mice with heterozygous and homozygous deletions of STAT5. In heterozygous STAT5 mutant mice, deficiencies in HSC/multipotent progenitors were reflected by decreased long-term repopulating activity. This reduction in repopulation function was mirrored in the reduced growth response to early-acting cytokines from sorted double mutant c-Kit(+)Lin(-)Sca-1(+) (KLS) cells. Importantly, in non-ablated newborn mice, the host steady-state engraftment ability was impaired by loss of Gab2 in heterozygous STAT5 mutant background. Fetal liver cells isolated from homozygous STAT5 mutant mice lacking Gab2 showed significant reduction in HSC number (KLS CD150(+)CD48(-)), reduced HSC survival, and dramatic loss of self-renewal potential as measured by serial transplantation.
Conclusions/significance: These data demonstrate new functions for Gab2 in hematopoiesis in a manner that is non-redundant with STAT5. Furthermore, important synergy between STAT5 and Gab2 was observed in HSC self-renewal, which might be exploited to optimize stem cell-based therapeutics.
Conflict of interest statement
Figures



Similar articles
-
Abnormal hematopoiesis in Gab2 mutant mice.Blood. 2007 Jul 1;110(1):116-24. doi: 10.1182/blood-2006-11-060707. Epub 2007 Mar 20. Blood. 2007. PMID: 17374739 Free PMC article.
-
Cell intrinsic defects in cytokine responsiveness of STAT5-deficient hematopoietic stem cells.Blood. 2002 Dec 1;100(12):3983-9. doi: 10.1182/blood-2002-05-1602. Epub 2002 Jul 25. Blood. 2002. PMID: 12393407
-
Conditional deletion of STAT5 in adult mouse hematopoietic stem cells causes loss of quiescence and permits efficient nonablative stem cell replacement.Blood. 2009 May 14;113(20):4856-65. doi: 10.1182/blood-2008-09-181107. Epub 2009 Mar 3. Blood. 2009. PMID: 19258595 Free PMC article.
-
STAT5 signaling in normal and pathologic hematopoiesis.Front Biosci. 2007 May 1;12:2807-20. doi: 10.2741/2274. Front Biosci. 2007. PMID: 17485261 Review.
-
Quantitative assessment of the stem cell self-renewal capacity.Ann N Y Acad Sci. 2001 Jun;938:18-24; discussion 24-5. doi: 10.1111/j.1749-6632.2001.tb03570.x. Ann N Y Acad Sci. 2001. PMID: 11458506 Review.
Cited by
-
GAB2--a scaffolding protein in cancer.Mol Cancer Res. 2012 Oct;10(10):1265-70. doi: 10.1158/1541-7786.MCR-12-0352. Epub 2012 Aug 7. Mol Cancer Res. 2012. PMID: 22871571 Free PMC article. Review.
-
STAT5 in hematopoietic stem cell biology and transplantation.JAKSTAT. 2013 Oct 1;2(4):e27159. doi: 10.4161/jkst.27159. Epub 2013 Nov 19. JAKSTAT. 2013. PMID: 24498540 Free PMC article. Review.
-
Oncogenic STAT Transcription Factors as Targets for Cancer Therapy: Innovative Strategies and Clinical Translation.Cancers (Basel). 2024 Mar 31;16(7):1387. doi: 10.3390/cancers16071387. Cancers (Basel). 2024. PMID: 38611065 Free PMC article. Review.
References
-
- Gu H, Pratt JC, Burakoff SJ, Neel BG. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2:729–740. - PubMed
-
- Nishida K, Yoshida Y, Itoh M, Fukada T, Ohtani T, et al. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood. 1999;93:1809–1816. - PubMed
-
- Crouin C, Arnaud M, Gesbert F, Camonis J, Bertoglio J. A yeast two-hybrid study of human p97/Gab2 interactions with its SH2 domain-containing binding partners. FEBS Lett. 2001;495:148–153. - PubMed
-
- Nishida K, Wang L, Morii E, Park SJ, Narimatsu M, et al. Requirement of Gab2 for mast cell development and KitL/c-Kit signalling. Blood. 2002;99:1866–1869. - PubMed
-
- Yu M, Luo J, Yang W, Wang Y, Mizuki M, et al. The scaffolding adapter Gab2, via Shp-2, regulates kit-evoked mast cell proliferation by activating the Rac/JNK pathway. J Biol Chem. 2006;281:28615–28626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

