TRAF5 is a downstream target of MAVS in antiviral innate immune signaling
- PMID: 20161788
- PMCID: PMC2820086
- DOI: 10.1371/journal.pone.0009172
TRAF5 is a downstream target of MAVS in antiviral innate immune signaling
Abstract
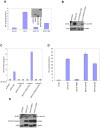
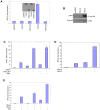
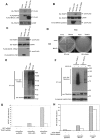
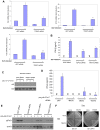
The recognition of nucleic acids by the innate immune system during viral infection results in the production of type I interferons and the activation of antiviral immune responses. The RNA helicases RIG-I and MDA-5 recognize distinct types of cytosolic RNA species and signal through the mitochondrial protein MAVS to stimulate the phosphorylation and activation of the transcription factors IRF3 and IRF7, thereby inducing type I interferon expression. Alternatively, the activation of NF-kappaB leads to proinflammatory cytokine production. The function of MAVS is dependent on both its C-terminal transmembrane (TM) domain and N-terminal caspase recruitment domain (CARD). The TM domain mediates MAVS dimerization in response to viral RNA, allowing the CARD to bind to and activate the downstream effector TRAF3. Notably, dimerization of the MAVS CARD alone is sufficient to activate IRF3, IRF7, and NF-kappaB. However, TRAF3-deficient cells display only a partial reduction in interferon production in response to RNA virus infection and are not defective in NF-kappaB activation. Here we find that the related ubiquitin ligase TRAF5 is a downstream target of MAVS that mediates both IRF3 and NF-kappaB activation. The TM domain of MAVS allows it to dimerize and thereby associate with TRAF5 and induce its ubiquitination in a CARD-dependent manner. Also, NEMO is recruited to the dimerized MAVS CARD domain in a TRAF3 and TRAF5-dependent manner. Thus, our findings reveal a possible function for TRAF5 in mediating the activation of IRF3 and NF-kappaB downstream of MAVS through the recruitment of NEMO. TRAF5 may be a key molecule in the innate response against viral infection.
Conflict of interest statement
Figures



Similar articles
-
MAVS self-association mediates antiviral innate immune signaling.J Virol. 2009 Apr;83(8):3420-8. doi: 10.1128/JVI.02623-08. Epub 2009 Feb 4. J Virol. 2009. PMID: 19193783 Free PMC article.
-
MAVS ubiquitination by the E3 ligase TRIM25 and degradation by the proteasome is involved in type I interferon production after activation of the antiviral RIG-I-like receptors.BMC Biol. 2012 May 24;10:44. doi: 10.1186/1741-7007-10-44. BMC Biol. 2012. PMID: 22626058 Free PMC article.
-
Dengue Virus Subverts Host Innate Immunity by Targeting Adaptor Protein MAVS.J Virol. 2016 Jul 27;90(16):7219-7230. doi: 10.1128/JVI.00221-16. Print 2016 Aug 15. J Virol. 2016. PMID: 27252539 Free PMC article.
-
Antiviral innate immunity pathways.Cell Res. 2006 Feb;16(2):141-7. doi: 10.1038/sj.cr.7310019. Cell Res. 2006. PMID: 16474426 Review.
-
Roles of tumor necrosis factor receptor associated factor 3 (TRAF3) and TRAF5 in immune cell functions.Immunol Rev. 2011 Nov;244(1):55-74. doi: 10.1111/j.1600-065X.2011.01055.x. Immunol Rev. 2011. PMID: 22017431 Free PMC article. Review.
Cited by
-
Emerging role of ubiquitination in antiviral RIG-I signaling.Microbiol Mol Biol Rev. 2012 Mar;76(1):33-45. doi: 10.1128/MMBR.05012-11. Microbiol Mol Biol Rev. 2012. PMID: 22390971 Free PMC article. Review.
-
Innate immune responses to RNA: sensing and signaling.Front Immunol. 2024 Jan 25;15:1287940. doi: 10.3389/fimmu.2024.1287940. eCollection 2024. Front Immunol. 2024. PMID: 38343534 Free PMC article. Review.
-
A functional C-terminal TRAF3-binding site in MAVS participates in positive and negative regulation of the IFN antiviral response.Cell Res. 2011 Jun;21(6):895-910. doi: 10.1038/cr.2011.2. Epub 2011 Jan 4. Cell Res. 2011. PMID: 21200404 Free PMC article.
-
MAVS recruits multiple ubiquitin E3 ligases to activate antiviral signaling cascades.Elife. 2013 Aug 14;2:e00785. doi: 10.7554/eLife.00785. Elife. 2013. PMID: 23951545 Free PMC article.
-
Negative regulation of MAVS-mediated innate immune response by ASC.Mol Cell Biochem. 2018 Aug;445(1-2):35-43. doi: 10.1007/s11010-017-3249-9. Epub 2017 Dec 26. Mol Cell Biochem. 2018. PMID: 29280086
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Hiscott J. Convergence of the NF-kappaB and IRF pathways in the regulation of the innate antiviral response. Cytokine Growth Factor Rev. 2007;18:483–490. - PubMed
-
- Hiscott J, Lin R, Nakhaei P, Paz S. MasterCARD: a priceless link to innate immunity. Trends Mol Med. 2006;12:53–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

