Ghrelin stimulation of growth hormone-releasing hormone neurons is direct in the arcuate nucleus
- PMID: 20161791
- PMCID: PMC2820089
- DOI: 10.1371/journal.pone.0009159
Ghrelin stimulation of growth hormone-releasing hormone neurons is direct in the arcuate nucleus
Abstract
Background: Ghrelin targets the arcuate nucleus, from where growth hormone releasing hormone (GHRH) neurones trigger GH secretion. This hypothalamic nucleus also contains neuropeptide Y (NPY) neurons which play a master role in the effect of ghrelin on feeding. Interestingly, connections between NPY and GHRH neurons have been reported, leading to the hypothesis that the GH axis and the feeding circuits might be co-regulated by ghrelin.
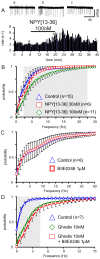
Principal findings: Here, we show that ghrelin stimulates the firing rate of identified GHRH neurons, in transgenic GHRH-GFP mice. This stimulation is prevented by growth hormone secretagogue receptor-1 antagonism as well as by U-73122, a phospholipase C inhibitor and by calcium channels blockers. The effect of ghrelin does not require synaptic transmission, as it is not antagonized by gamma-aminobutyric acid, glutamate and NPY receptor antagonists. In addition, this hypothalamic effect of ghrelin is independent of somatostatin, the inhibitor of the GH axis, since it is also found in somatostatin knockout mice. Indeed, ghrelin does not modify synaptic currents of GHRH neurons. However, ghrelin exerts a strong and direct depolarizing effect on GHRH neurons, which supports their increased firing rate.
Conclusion: Thus, GHRH neurons are a specific target for ghrelin within the brain, and not activated secondary to altered activity in feeding circuits. These results support the view that ghrelin related therapeutic approaches could be directed separately towards GH deficiency or feeding disorders.
Conflict of interest statement
Figures







Similar articles
-
Growth hormone-releasing hormone (GHRH) neurons in the arcuate nucleus (Arc) of the hypothalamus are decreased in transgenic rats whose expression of ghrelin receptor is attenuated: Evidence that ghrelin receptor is involved in the up-regulation of GHRH expression in the arc.Endocrinology. 2006 Sep;147(9):4093-103. doi: 10.1210/en.2005-1619. Epub 2006 May 25. Endocrinology. 2006. PMID: 16728494
-
Ghrelin and obestatin modulate growth hormone-releasing hormone release and synaptic inputs onto growth hormone-releasing hormone neurons.Eur J Neurosci. 2011 Sep;34(5):732-44. doi: 10.1111/j.1460-9568.2011.07787.x. Epub 2011 Jul 21. Eur J Neurosci. 2011. PMID: 21777303
-
A natural variant of obestatin, Q90L, inhibits ghrelin's action on food intake and GH secretion and targets NPY and GHRH neurons in mice.PLoS One. 2012;7(12):e51135. doi: 10.1371/journal.pone.0051135. Epub 2012 Dec 10. PLoS One. 2012. PMID: 23251435 Free PMC article.
-
Clinical pharmacology of human growth hormone and its secretagogues.Curr Drug Targets Immune Endocr Metabol Disord. 2002 Apr;2(1):27-52. Curr Drug Targets Immune Endocr Metabol Disord. 2002. PMID: 12477295 Review.
-
Growth hormone secretagogues and hypothalamic networks.Endocrine. 2001 Feb;14(1):1-8. doi: 10.1385/ENDO:14:1:001. Endocrine. 2001. PMID: 11322489 Review.
Cited by
-
Electrophysiology of Hypothalamic Magnocellular Neurons In vitro: A Rhythmic Drive in Organotypic Cultures and Acute Slices.Front Neurosci. 2016 Mar 31;10:109. doi: 10.3389/fnins.2016.00109. eCollection 2016. Front Neurosci. 2016. PMID: 27065780 Free PMC article.
-
Down-regulation of ghrelin receptors on dopaminergic neurons in the substantia nigra contributes to Parkinson's disease-like motor dysfunction.Mol Brain. 2018 Feb 20;11(1):6. doi: 10.1186/s13041-018-0349-8. Mol Brain. 2018. PMID: 29458391 Free PMC article.
-
Clustered regularly interspaced short palindromic repeats as an advanced treatment for Parkinson's disease.Brain Behav. 2021 Aug;11(8):e2280. doi: 10.1002/brb3.2280. Epub 2021 Jul 21. Brain Behav. 2021. PMID: 34291612 Free PMC article. Review.
-
Actions of NPY, and its Y1 and Y2 receptors on pulsatile growth hormone secretion during the fed and fasted state.J Neurosci. 2014 Dec 3;34(49):16309-19. doi: 10.1523/JNEUROSCI.4622-13.2014. J Neurosci. 2014. PMID: 25471570 Free PMC article.
-
Metabolic responses to exogenous ghrelin in obesity and early after Roux-en-Y gastric bypass in humans.Diabetes Obes Metab. 2017 Sep;19(9):1267-1275. doi: 10.1111/dom.12952. Epub 2017 May 31. Diabetes Obes Metab. 2017. PMID: 28345790 Free PMC article. Clinical Trial.
References
-
- Farhy LS, Bowers CY, Veldhuis JD. Model-projected mechanistic bases for sex differences in growth hormone regulation in humans. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1577–93. - PubMed
-
- Bowers CY, Momany FA, Reynolds GA, Hong A. On the in vitro and in vivo activity of a new synthetic hexapeptide that acts on the pituitary to specifically release growth hormone. Endocrinology. 1984;114:1537–45. - PubMed
-
- Smith RG. Development of growth hormone secretagogues. Endocr Rev. 2005;26:346–360. - PubMed
-
- Nakazato M, Murakami N, Date Y, Kojima M, Matsuo M, et al. A role for ghrelin in the central regulation of feeding. Nature London. 2001;409:194–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

