HTRF: A technology tailored for drug discovery - a review of theoretical aspects and recent applications
- PMID: 20161833
- PMCID: PMC2802762
- DOI: 10.2174/1875397300903010022
HTRF: A technology tailored for drug discovery - a review of theoretical aspects and recent applications
Abstract
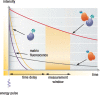
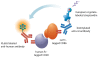
HTRF (Homogeneous Time Resolved Fluorescence) is the most frequently used generic assay technology to measure analytes in a homogenous format, which is the ideal platform used for drug target studies in high-throughput screening (HTS). This technology combines fluorescence resonance energy transfer technology (FRET) with time-resolved measurement (TR). In TR-FRET assays, a signal is generated through fluorescent resonance energy transfer between a donor and an acceptor molecule when in close proximity to each other. Buffer and media interference is dramatically reduced by dual-wavelength detection, and the final signal is proportional to the extent of product formation. The HTRF assay is usually sensitive and robust that can be miniaturized into the 384 and 1536-well plate formats. This assay technology has been applied to many antibody-based assays including GPCR signaling (cAMP and IP-One), kinases, cytokines and biomarkers, bioprocess (antibody and protein production), as well as the assays for protein-protein, proteinpeptide, and protein-DNA/RNA interactions.Since its introduction to the drug-screening world over ten years ago, researchers have used HTRF to expedite the study of GPCRs, kinases, new biomarkers, protein-protein interactions, and other targets of interest. HTRF has also been utilized as an alternative method for bioprocess monitoring. The first-generation HTRF technology, which uses Europium cryptate as a fluorescence donor to monitor reactions between biomolecules, was extended in 2008 through the introduction of a second-generation donor, Terbium cryptate (Tb), enhancing screening performance. Terbium cryptate possesses different photophysical properties compared to Europium, including increased quantum yield and a higher molar extinction coefficient. In addition to being compatible with the same acceptor fluorophors used with Europium, it can serve as a donor fluorophore to green-emitting fluors because it has multiple emission peaks including one at 490 nm. Moreover, all Terbium HTRF assays can be read on the same HTRF-compatible instruments as Europium HTRF assays.Overall, HTRF is a highly sensitive, robust technology for the detection of molecular interactions in vitro and is widely used for primary and secondary screening phases of drug development. This review addresses the general principles of HTRF and its current applications in drug discovery.
Keywords: Biomarker; Bioprocess.; GPCR; HTRF; Kinase; TR-FRET.
Figures

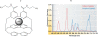

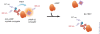
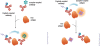

References
-
- Mathis G. Probing molecular interactions with homogeneous techniques based on rare earth cryptates and fluorescence energy transfer. Clin Chem. 1995;41(9):1391–7. - PubMed
-
- Selvin PR. Principles and biophysical applications of lanthanide-based probes. Annu Rev Biophys Biomol Struct. 2002;31:275–302. - PubMed
-
- Mathis G. HTRF(R) Technology. J Biomol Screen. 1999;4(6):309–14. - PubMed
-
- Bazin H, Trinquet E, Mathis G. Time resolved amplification of cryptate emission: a versatile technology to trace biomolecular interactions. J Biotechnol. 2002;82(3):233–50. - PubMed
-
- Alpha B, Ballardini R, Balzani V, Lehn JM, Perathoner S, Sabbatini N. Antenna effect in luminescent lanthanide cryptate: a photophysical study. Photochem Photobiol. 1990;52(2):299–306.
LinkOut - more resources
Full Text Sources
Other Literature Sources
