A homogenous luminescent proximity assay for 14-3-3 interactions with both phosphorylated and nonphosphorylated client peptides
- PMID: 20161842
- PMCID: PMC2803432
- DOI: 10.2174/1875397300802010040
A homogenous luminescent proximity assay for 14-3-3 interactions with both phosphorylated and nonphosphorylated client peptides
Abstract
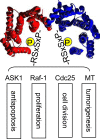
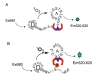
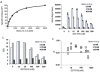
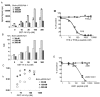
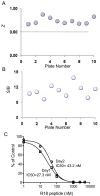
The 14-3-3 proteins are a family of dimeric eukaryotic proteins that mediate both phosphorylation-dependent and -independent protein-protein interactions. Through these interactions, 14-3-3 proteins participate in the regulation of a wide range of cellular processes, including cell proliferation, cell cycle progression, and apoptosis. Because of their fundamental importance, 14-3-3 proteins have also been implicated in a variety of diseases, including cancer and neurodegenerative disorders. In order to monitor 14-3-3/client protein interactions for the discovery of small molecule 14-3-3 modulators, we have designed and optimized 14-3-3 protein binding assays based on the amplified luminescent proximity homogeneous assay (AlphaScreen) technology. Using the interaction of 14-3-3 with a phosphorylated Raf-1 peptide and a nonphosphorylated R18 peptide as model systems, we have established homogenous "add-and-measure" high-throughput screening assays. Both assays achieved robust performance with S/B ratios above 7 and Z' factors above 0.7. Application of the known antagonistic peptides in our studies further validated the assay for screening of chemical compound libraries to identify small molecules that can modulate 14-3-3 protein-protein interactions.
Keywords: 14-3-3; AlphaScreen; HTS.; protein-protein interaction.
Figures
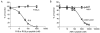

References
-
- Fu H, Subramanian RR, Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–47. - PubMed
-
- Muslin AJ, Xing H. 14-3-3 proteins: regulation of subcellular localization by molecular interference. Cell Signal. 2000;12(11-12):703–9. - PubMed
-
- Yaffe MB. How do 14-3-3 proteins work?-- Gatekeeper phosphorylation and the molecular anvil hypothesis. FEBS Lett. 2002;513(1):53–7. - PubMed
-
- Aitken A. 14-3-3 proteins: a historic overview. Semin Cancer Biol. 2006;16(3):162–72. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
