Inhibitory activity of marine sponge-derived natural products against parasitic protozoa
- PMID: 20161970
- PMCID: PMC2817922
- DOI: 10.3390/md8010047
Inhibitory activity of marine sponge-derived natural products against parasitic protozoa
Abstract
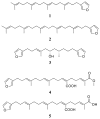
In this study, thirteen sponge-derived terpenoids, including five linear furanoterpenes: furospinulosin-1 (1), furospinulosin-2 (2), furospongin-1 (3), furospongin-4 (4), and demethylfurospongin-4 (5); four linear meroterpenes: 2-(hexaprenylmethyl)-2-methylchromenol (6), 4-hydroxy-3-octaprenylbenzoic acid (7), 4-hydroxy-3-tetraprenyl-phenylacetic acid (8), and heptaprenyl-p-quinol (9); a linear triterpene, squalene (10); two spongian-type diterpenes dorisenone D (11) and 11 beta-acetoxyspongi-12-en-16-one (12); a scalarane-type sesterterpene; 12-epi-deoxoscalarin (13), as well as an indole alkaloid, tryptophol (14) were screened for their in vitro activity against four parasitic protozoa; Trypanosoma brucei rhodesiense, Trypanosoma cruzi, Leishmania donovani and Plasmodium falciparum. Cytotoxic potential of the compounds on mammalian cells was also assessed. All compounds were active against T. brucei rhodesiense, with compound 8 being the most potent (IC(50) 0.60 microg/mL), whereas 9 and 12 were the most active compounds against T. cruzi, with IC(50) values around 4 microg/mL. Compound 12 showed the strongest leishmanicidal activity (IC(50) 0.75 microg/mL), which was comparable to that of miltefosine (IC(50) 0.20 microg/mL). The best antiplasmodial effect was exerted by compound 11 (IC(50) 0.43 microg/mL), followed by compounds 7, 10, and 12 with IC(50) values around 1 microg/mL. Compounds 9, 11 and 12 exhibited, besides their antiprotozoal activity, also some cytotoxicity, whereas all other compounds had low or no cytotoxicity towards the mammalian cell line. This is the first report of antiprotozoal activity of marine metabolites 1-14, and points out the potential of marine sponges in discovery of new antiprotozoal lead compounds.
Keywords: Ircinia; Leishmania; Plasmodium; Spongia; Trypanosoma; antiprotozoal activity; marine sponge.
Figures
Similar articles
-
Evaluation of Turkish seaweeds for antiprotozoal, antimycobacterial and cytotoxic activities.Phytother Res. 2011 May;25(5):778-83. doi: 10.1002/ptr.3330. Epub 2010 Nov 12. Phytother Res. 2011. PMID: 21520472
-
Turkish freshwater and marine macrophyte extracts show in vitro antiprotozoal activity and inhibit FabI, a key enzyme of Plasmodium falciparum fatty acid biosynthesis.Phytomedicine. 2006 Jun;13(6):388-93. doi: 10.1016/j.phymed.2005.10.010. Epub 2006 May 11. Phytomedicine. 2006. PMID: 16697632
-
Anti-Protozoal Activities of Cembrane-Type Diterpenes from Vietnamese Soft Corals.Molecules. 2015 Jul 8;20(7):12459-68. doi: 10.3390/molecules200712459. Molecules. 2015. PMID: 26184133 Free PMC article.
-
The Role of Spongia sp. in the Discovery of Marine Lead Compounds.Mar Drugs. 2016 Jul 23;14(8):139. doi: 10.3390/md14080139. Mar Drugs. 2016. PMID: 27455286 Free PMC article. Review.
-
Bioprospecting Sponge-Associated Microbes for Antimicrobial Compounds.Mar Drugs. 2016 May 2;14(5):87. doi: 10.3390/md14050087. Mar Drugs. 2016. PMID: 27144573 Free PMC article. Review.
Cited by
-
Nitrotriazole-based acetamides and propanamides with broad spectrum antitrypanosomal activity.Eur J Med Chem. 2016 Nov 10;123:895-904. doi: 10.1016/j.ejmech.2016.08.002. Epub 2016 Aug 9. Eur J Med Chem. 2016. PMID: 27543881 Free PMC article.
-
Novel 3-nitro-1H-1,2,4-triazole-based compounds as potential anti-Chagasic drugs: in vivo studies.Future Med Chem. 2013 Oct;5(15):1763-76. doi: 10.4155/fmc.13.108. Future Med Chem. 2013. PMID: 24144412 Free PMC article.
-
Two trypanocidal dipeptides from the roots of Zapoteca portoricensis (Fabaceae).Molecules. 2014 Apr 25;19(5):5470-7. doi: 10.3390/molecules19055470. Molecules. 2014. PMID: 24776813 Free PMC article.
-
Acid-Induced Rearrangement of Epoxygermacranolides: Synthesis of Furanoheliangolides and Cadinanes from Nobilin.Molecules. 2017 Dec 18;22(12):2252. doi: 10.3390/molecules22122252. Molecules. 2017. PMID: 29258233 Free PMC article.
-
Unusual derivatives from Hypericum scabrum.Sci Rep. 2021 Jan 14;10(1):22181. doi: 10.1038/s41598-020-79305-y. Sci Rep. 2021. PMID: 33446755 Free PMC article.
References
-
- WHO. World malaria report. World Health Organization; 2008. [(Accessed on 20 November 2009)]. Available online http://malaria.who.int/wmr2008/malaria2008.pdf.
-
- D’Alessandro U. Existing antimalarial agents and malaria-treatment strategies. Exp Opin Pharmacother. 2009;10:1291–1306. - PubMed
-
- Brun R, Blum J, Chappuis F, Burri C. Human African trypanosomiasis. Lancet. 2009 in press. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials