Calyculins and related marine natural products as serine-threonine protein phosphatase PP1 and PP2A inhibitors and total syntheses of calyculin A, B, and C
- PMID: 20161975
- PMCID: PMC2817927
- DOI: 10.3390/md80100122
Calyculins and related marine natural products as serine-threonine protein phosphatase PP1 and PP2A inhibitors and total syntheses of calyculin A, B, and C
Abstract
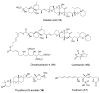
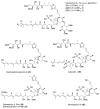
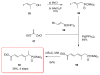
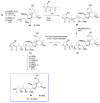
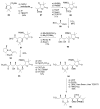
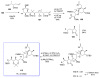
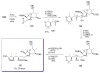
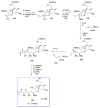
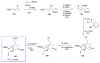
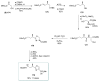
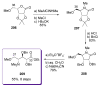
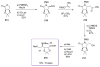
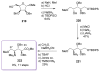
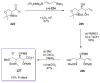
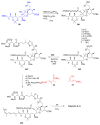
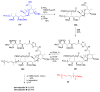
Calyculins, highly cytotoxic polyketides, originally isolated from the marine sponge Discodermia calyx by Fusetani and co-workers, belong to the lithistid sponges group. These molecules have become interesting targets for cell biologists and synthetic organic chemists. The serine/threonine protein phosphatases play an essential role in the cellular signalling, metabolism, and cell cycle control. Calyculins express potent protein phosphatase 1 and 2A inhibitory activity, and have therefore become valuable tools for cellular biologists studying intracellular processes and their control by reversible phosphorylation. Calyculins might also play an important role in the development of several diseases such as cancer, neurodegenerative diseases, and type 2-diabetes mellitus. The fascinating structures of calyculins have inspired various groups of synthetic organic chemists to develop total syntheses of the most abundant calyculins A and C. However, with fifteen chiral centres, a cyano-capped tetraene unit, a phosphate-bearing spiroketal, an anti, anti, anti dipropionate segment, an alpha-chiral oxazole, and a trihydroxylated gamma-amino acid, calyculins reach versatility that only few natural products can surpass, and truly challenge modern chemists' asymmetric synthesis skills.
Keywords: marine natural products; protein phosphatase inhibitors; total synthesis.
Figures

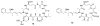
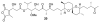

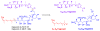
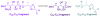



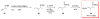
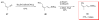






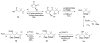


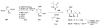

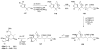
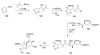

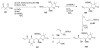

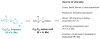
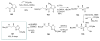


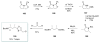
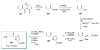




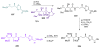

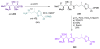
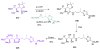


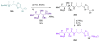



Similar articles
-
Carcinogenic aspects of protein phosphatase 1 and 2A inhibitors.Prog Mol Subcell Biol. 2009;46:221-54. doi: 10.1007/978-3-540-87895-7_8. Prog Mol Subcell Biol. 2009. PMID: 19184590 Review.
-
Inhibition of protein phosphatase-1 by clavosines A and B. Novel members of the calyculin family of toxins.J Biol Chem. 2000 Feb 11;275(6):4192-8. doi: 10.1074/jbc.275.6.4192. J Biol Chem. 2000. PMID: 10660582
-
Zoledronic acid in combination with serine/threonine phosphatase inhibitors induces enhanced cytotoxicity and apoptosis in hormone-refractory prostate cancer cell lines by decreasing the activities of PP1 and PP2A.BJU Int. 2012 Dec;110(11 Pt C):E1147-54. doi: 10.1111/j.1464-410X.2012.11392.x. Epub 2012 Aug 9. BJU Int. 2012. PMID: 22882676
-
Protein phosphatases 1 and 2A and their naturally occurring inhibitors: current topics in smooth muscle physiology and chemical biology.J Physiol Sci. 2018 Jan;68(1):1-17. doi: 10.1007/s12576-017-0556-6. Epub 2017 Jul 5. J Physiol Sci. 2018. PMID: 28681362 Free PMC article. Review.
-
PP1/PP2A phosphatase inhibition-induced metaplasticity in protein synthesis blocker-treated hippocampal slices: LTP and LTD, or There and Back again.Biochem Biophys Res Commun. 2021 Jun 18;558:64-70. doi: 10.1016/j.bbrc.2021.04.061. Epub 2021 Apr 23. Biochem Biophys Res Commun. 2021. PMID: 33901925
Cited by
-
Muscarinic-activated potassium current mediates the negative chronotropic effect of pilocarpine on the rabbit sinoatrial node.Pflugers Arch. 2011 Aug;462(2):235-43. doi: 10.1007/s00424-011-0962-1. Epub 2011 Apr 13. Pflugers Arch. 2011. PMID: 21487692
-
Renal AT2 Receptors Mediate Natriuresis via Protein Phosphatase PP2A.Circ Res. 2022 Jan 7;130(1):96-111. doi: 10.1161/CIRCRESAHA.121.319519. Epub 2021 Nov 19. Circ Res. 2022. PMID: 34794320 Free PMC article.
-
Phosphatases Decrease Water and Urea Permeability in Rat Inner Medullary Collecting Ducts.Int J Mol Sci. 2023 Mar 31;24(7):6537. doi: 10.3390/ijms24076537. Int J Mol Sci. 2023. PMID: 37047509 Free PMC article.
-
Crosstalk of the EphA2 receptor with a serine/threonine phosphatase suppresses the Akt-mTORC1 pathway in cancer cells.Cell Signal. 2011 Jan;23(1):201-12. doi: 10.1016/j.cellsig.2010.09.004. Epub 2010 Sep 15. Cell Signal. 2011. PMID: 20837138 Free PMC article.
-
The Phosphatase Inhibitor Calyculin-A Impairs Clot Retraction, Platelet Activation, and Thrombin Generation.Biomed Res Int. 2017;2017:9795271. doi: 10.1155/2017/9795271. Epub 2017 Jun 7. Biomed Res Int. 2017. PMID: 28680886 Free PMC article.
References
-
- Nakao Y, Fusetani N. Enzyme inhibitors from marine invertebrates. J Nat Prod. 2007;70:689–710. - PubMed
-
- Barford D, Das AK, Egloff M-P. The strucrure and mechanism of protein phosphatases: Insights into catalysis and regulation. Annu Rev Biophys Biomol Struct. 1998;27:133–164. - PubMed
-
- Cohen PTW. Novel protein serine/threonine phosphatases: Variety is the spice of life. Trends Biochem Sci. 1997;22:245–251. - PubMed
-
- McCluskey A, Sim ATR, Sakoff JA. Serine-Threonine protein phosphatase inhibitors: Development of potential therapeutic strategies. J Med Chem. 2002;45:1151–1175. - PubMed
-
- Schönthal AH. Role of serine/threonine protein phosphatase 2A in cancer. Cancer Lett. 2001;170:1–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
