Glycine-spacers influence functional motifs exposure and self-assembling propensity of functionalized substrates tailored for neural stem cell cultures
- PMID: 20162033
- PMCID: PMC2821182
- DOI: 10.3389/neuro.16.001.2010
Glycine-spacers influence functional motifs exposure and self-assembling propensity of functionalized substrates tailored for neural stem cell cultures
Abstract
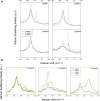
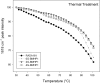
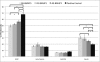
The understanding of phenomena involved in the self-assembling of bio-inspired biomaterials acting as three-dimensional scaffolds for regenerative medicine applications is a necessary step to develop effective therapies in neural tissue engineering. We investigated the self-assembled nanostructures of functionalized peptides featuring four, two or no glycine-spacers between the self-assembly sequence RADA16-I and the functional biological motif PFSSTKT. The effectiveness of their biological functionalization was assessed via in vitro experiments with neural stem cells (NSCs) and their molecular assembly was elucidated via atomic force microscopy, Raman and Fourier Transform Infrared spectroscopy. We demonstrated that glycine-spacers play a crucial role in the scaffold stability and in the exposure of the functional motifs. In particular, a glycine-spacer of four residues leads to a more stable nanostructure and to an improved exposure of the functional motif. Accordingly, the longer spacer of glycines, the more effective is the functional motif in both eliciting NSCs adhesion, improving their viability and increasing their differentiation. Therefore, optimized designing strategies of functionalized biomaterials may open, in the near future, new therapies in tissue engineering and regenerative medicine.
Keywords: AFM; FTIR; Micro-Raman; biomaterial; nanostructure; neural stem cell.
Figures
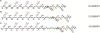



Similar articles
-
BMHP1-derived self-assembling peptides: hierarchically assembled structures with self-healing propensity and potential for tissue engineering applications.ACS Nano. 2011 Mar 22;5(3):1845-59. doi: 10.1021/nn102663a. Epub 2011 Feb 11. ACS Nano. 2011. PMID: 21314189
-
Branched peptides integrate into self-assembled nanostructures and enhance biomechanics of peptidic hydrogels.Acta Biomater. 2018 Jan 15;66:258-271. doi: 10.1016/j.actbio.2017.11.026. Epub 2017 Nov 8. Acta Biomater. 2018. PMID: 29128535
-
Biocompatibility of functionalized designer self-assembling nanofiber scaffolds containing FRM motif for neural stem cells.J Biomed Mater Res A. 2014 May;102(5):1286-93. doi: 10.1002/jbm.a.34804. Epub 2013 Jun 4. J Biomed Mater Res A. 2014. PMID: 23703883
-
Self-assembling peptides: from bio-inspired materials to bone regeneration.J Dent Res. 2008 Jul;87(7):606-16. doi: 10.1177/154405910808700710. J Dent Res. 2008. PMID: 18573978 Review.
-
Biomimetic Self-Assembling Peptide Hydrogels for Tissue Engineering Applications.Adv Exp Med Biol. 2018;1064:297-312. doi: 10.1007/978-981-13-0445-3_18. Adv Exp Med Biol. 2018. PMID: 30471040 Review.
Cited by
-
Identification of a binding site of the human immunodeficiency virus envelope protein gp120 to neuronal-specific tubulin.J Neurochem. 2016 Apr;137(2):287-98. doi: 10.1111/jnc.13557. Epub 2016 Mar 15. J Neurochem. 2016. PMID: 26826352 Free PMC article.
-
Mechano-Transduction Signals Derived from Self-Assembling Peptide Nanofibers Containing Long Motif of Laminin Influence Neurogenesis in In-Vitro and In-Vivo.Mol Neurobiol. 2017 May;54(4):2483-2496. doi: 10.1007/s12035-016-9836-z. Epub 2016 Mar 16. Mol Neurobiol. 2017. PMID: 26984600
-
Self-Assembling Peptide Nanofiber Containing Long Motif of Laminin Induces Neural Differentiation, Tubulin Polymerization, and Neurogenesis: In Vitro, Ex Vivo, and In Vivo Studies.Mol Neurobiol. 2016 Oct;53(8):5288-99. doi: 10.1007/s12035-015-9448-z. Epub 2015 Oct 1. Mol Neurobiol. 2016. PMID: 26427854
-
Enhancement of Neural Stem Cell Survival, Proliferation, Migration, and Differentiation in a Novel Self-Assembly Peptide Nanofibber Scaffold.Mol Neurobiol. 2017 Dec;54(10):8050-8062. doi: 10.1007/s12035-016-0295-3. Epub 2016 Nov 23. Mol Neurobiol. 2017. PMID: 27878763
-
Differentiation of human neural progenitor cells in functionalized hydrogel matrices.Biores Open Access. 2012 Jan;1(1):16-24. doi: 10.1089/biores.2012.0209. Biores Open Access. 2012. PMID: 23515105 Free PMC article.
References
-
- Davis M. E., Hsieh P. C., Takahashi T., Song Q., Zhang S., Kamm R. D., Grodzinsky A. J., Anversa P., Lee R. T. (2006). Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc. Natl. Acad. Sci. U.S.A. 103, 8155–816010.1073/pnas.0602877103 - DOI - PMC - PubMed
-
- De Gelder J., De Gussem K., Vandenabeele P., Moens L. (2007). Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 38, 1133–114710.1002/jrs.1734 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

