Characterization of a stretch-activated potassium channel in chondrocytes
- PMID: 20162564
- PMCID: PMC2883078
- DOI: 10.1002/jcp.22075
Characterization of a stretch-activated potassium channel in chondrocytes
Abstract
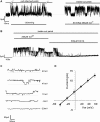
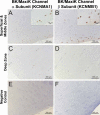
Chondrocytes possess the capacity to transduce load-induced mechanical stimuli into electrochemical signals. The aim of this study was to functionally characterize an ion channel activated in response to membrane stretch in isolated primary equine chondrocytes. We used patch-clamp electrophysiology to functionally characterize this channel and immunohistochemistry to examine its distribution in articular cartilage. In cell-attached patch experiments, the application of negative pressures to the patch pipette (in the range of 20-200 mmHg) activated ion channel currents in six of seven patches. The mean activated current was 45.9 +/- 1.1 pA (n = 4) at a membrane potential of 33 mV (cell surface area approximately 240 microm(2)). The mean slope conductance of the principal single channels resolved within the total stretch-activated current was 118 +/- 19 pS (n = 6), and reversed near the theoretical potassium equilibrium potential, E(K+), suggesting it was a high-conductance potassium channel. Activation of these high-conductance potassium channels was inhibited by extracellular TEA (K(d) approx. 900 microM) and iberiotoxin (K(d) approx. 40 nM). This suggests that the current was largely carried by BK-like potassium (MaxiK) channels. To further characterize these BK-like channels, we used inside-out patches of chondrocyte membrane: we found these channels to be activated by elevation in bath calcium concentration. Immunohistochemical staining of equine cartilage samples with polyclonal antibodies to the alpha1- and beta1-subunits of the BK channel revealed positive immunoreactivity for both subunits in superficial zone chondrocytes. These experiments support the hypothesis that functional BK channels are present in chondrocytes and may be involved in mechanotransduction and chemotransduction.
Figures

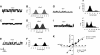
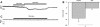
Similar articles
-
Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: Potential therapeutic targets for osteoarthritis.Channels (Austin). 2021 Dec;15(1):339-359. doi: 10.1080/19336950.2021.1903184. Channels (Austin). 2021. PMID: 33775217 Free PMC article. Review.
-
Characterisation of large-conductance calcium-activated potassium channels (BK(Ca)) in human NT2-N cells.Brain Res. 2007 Jan 19;1129(1):15-25. doi: 10.1016/j.brainres.2006.10.060. Epub 2006 Dec 6. Brain Res. 2007. PMID: 17156763
-
Properties of large conductance calcium-activated potassium channels in pyramidal neurons from the hippocampal CA1 region of adult rats.Jpn J Physiol. 2001 Dec;51(6):725-31. doi: 10.2170/jjphysiol.51.725. Jpn J Physiol. 2001. PMID: 11846964
-
Characterization of voltage-and Ca2+-activated K+ channels in rat dorsal root ganglion neurons.J Cell Physiol. 2007 Aug;212(2):348-57. doi: 10.1002/jcp.21007. J Cell Physiol. 2007. PMID: 17523149
-
An unexpected journey: conceptual evolution of mechanoregulated potassium transport in the distal nephron.Am J Physiol Cell Physiol. 2016 Feb 15;310(4):C243-59. doi: 10.1152/ajpcell.00328.2015. Epub 2015 Dec 2. Am J Physiol Cell Physiol. 2016. PMID: 26632600 Free PMC article. Review.
Cited by
-
Switch of voltage-gated K+ channel expression in the plasma membrane of chondrogenic cells affects cytosolic Ca2+-oscillations and cartilage formation.PLoS One. 2011;6(11):e27957. doi: 10.1371/journal.pone.0027957. Epub 2011 Nov 21. PLoS One. 2011. PMID: 22132179 Free PMC article.
-
Potassium channels in articular chondrocytes.Channels (Austin). 2012 Nov-Dec;6(6):416-25. doi: 10.4161/chan.22340. Epub 2012 Oct 12. Channels (Austin). 2012. PMID: 23064164 Free PMC article. Review.
-
Two-pore domain K⁺ channels regulate membrane potential of isolated human articular chondrocytes.J Physiol. 2011 Nov 1;589(Pt 21):5071-89. doi: 10.1113/jphysiol.2011.210757. Epub 2011 Sep 12. J Physiol. 2011. PMID: 21911614 Free PMC article.
-
Physicochemical and biomechanical stimuli in cell-based articular cartilage repair.Curr Rheumatol Rep. 2015 Mar;17(3):22. doi: 10.1007/s11926-014-0493-9. Curr Rheumatol Rep. 2015. PMID: 25828845 Free PMC article. Review.
-
Mechanosensory and mechanotransductive processes mediated by ion channels in articular chondrocytes: Potential therapeutic targets for osteoarthritis.Channels (Austin). 2021 Dec;15(1):339-359. doi: 10.1080/19336950.2021.1903184. Channels (Austin). 2021. PMID: 33775217 Free PMC article. Review.
References
-
- Archer CW, Francis-West P. The chondrocyte. Int J Biochem Cell Biol. 2003;35:401–404. - PubMed
-
- Barrett-Jolley R, Pyner S, Coote JH. Measurement of voltage-gated potassium currents in identified spinally-projecting sympathetic neurones of the paraventricular nucleus. J Neurosci Methods. 2000;102:25–33. - PubMed
-
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

