Hepatitis C virus core protein interacts with fibrinogen-beta and attenuates cytokine stimulated acute-phase response
- PMID: 20162731
- PMCID: PMC5837823
- DOI: 10.1002/hep.23502
Hepatitis C virus core protein interacts with fibrinogen-beta and attenuates cytokine stimulated acute-phase response
Abstract
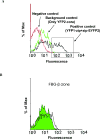
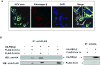
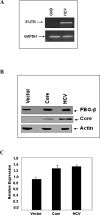
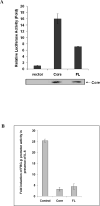
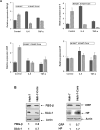
Fibrinogen-beta (FBG-beta), an important acute-phase protein (APP), is generated by the liver as a target for inflammatory mediators. Here we identified FBG-beta as a hepatitis C virus (HCV) core interacting protein by screening a human liver complementary DNA (cDNA) library using mammalian two-hybrid analysis. An association between FBG-beta and HCV core protein was verified by confocal microscopy and coimmunoprecipitation from the transfected human hepatocyte (Huh-7) cell line. HCV core or genomic RNA transfected Huh-7 cells modestly increased FBG-beta protein expression when compared to the basal level in control hepatocytes. Transfection of HCV core or full-length (FL) gene into Huh-7 cells up-regulated basal FBG-beta promoter activity. Exogenous addition of IL-6 stimulates FBG-beta promoter activity in hepatocytes. However, ectopic expression of HCV core or FL in hepatocytes inhibited IL-6-stimulated FBG-beta promoter activation. Inhibition of endogenous FBG-beta expression following introduction of small interfering RNA (siRNA) into cells displayed a gain of function of promoter regulation by HCV core protein. Further studies suggested that HCV core gene expression in stable transfectants of Huh-7 cells resulted in a basal up-regulation of FBG-beta and other APPs. However, treatment with cytokines, interleukin-6 (IL-6), or tumor necrosis factor-alpha repressed FBG-beta and other acute-phase response (APR) genes.
Conclusion: Our results reveal that the core/FBG-beta interaction may act as a regulatory feedback, allowing repression of IL-6-stimulated APR genes. Together, these data suggested a network of interactions between HCV core and the hepatic APR genes, and may contribute to impaired innate immunity for viral persistence.
Figures

References
-
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. - PubMed
-
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16. - PubMed
-
- Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. - PubMed
-
- Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain, Behav & Immun. 2003;17:350–64. - PubMed
-
- Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc. Natl. Acad. Sci. 1987;84:7251–7255. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
