Effect of HEPES buffer on the uptake and transport of P-glycoprotein substrates and large neutral amino acids
- PMID: 20163160
- PMCID: PMC2849840
- DOI: 10.1021/mp900193e
Effect of HEPES buffer on the uptake and transport of P-glycoprotein substrates and large neutral amino acids
Abstract
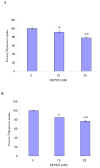
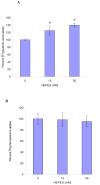
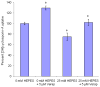
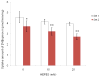
HEPES has been widely employed as an organic buffer agent in cell culture medium as well as uptake and transport experiments in vitro. However, concentrations of HEPES used in such studies vary from one laboratory to another. In this study, we investigated the effect of HEPES on the uptake and bidirectional transport of P-gp substrates employing both Caco-2 and MDCK-MDR1 cells. ATP-dependent uptake of glutamic acid was also examined. ATP production was further quantified applying ATP Determination Kit. An addition of HEPES to the growth and incubation media significantly altered the uptake and transport of P-gp substrates in both Caco-2 and MDCK-MDR1 cells. Uptake of P-gp substrates substantially diminished as the HEPES concentration was raised to 25 mM. Bidirectional (A-B and B-A) transport studies revealed that permeability ratio of P(appB-A) to P(appA-B) in the presence of 25 mM HEPES was significantly higher than control. The uptake of phenylalanine is an ATP-independent process, whereas the accumulation of glutamic acid is ATP-dependent. While phenylalanine uptake remained unchanged, glutamic acid uptake was elevated with the addition of HEPES. Verapamil is an inhibitor of P-gp mediated uptake; elevation of cyclosporine uptake in the presence of 5 muM verapamil was compromised by the presence of 25 mM HEPES. The results of ATP assay indicated that HEPES stimulated the production of ATP. This study suggests that the addition of HEPES in the medium modulated the energy dependent efflux and uptake processes. The effect of HEPES on P-gp mediated drug efflux and transport may provide some mechanistic insight into possible reasons for inconsistencies in the results reported from various laboratories.
Figures




Similar articles
-
Transport inhibition of digoxin using several common P-gp expressing cell lines is not necessarily reporting only on inhibitor binding to P-gp.PLoS One. 2013 Aug 16;8(8):e69394. doi: 10.1371/journal.pone.0069394. eCollection 2013. PLoS One. 2013. PMID: 23976943 Free PMC article.
-
Functional assessment of multiple P-glycoprotein (P-gp) probe substrates: influence of cell line and modulator concentration on P-gp activity.Drug Metab Dispos. 2005 Nov;33(11):1679-87. doi: 10.1124/dmd.105.005421. Epub 2005 Aug 10. Drug Metab Dispos. 2005. PMID: 16093365
-
Role of P-glycoprotein in the intestinal absorption of tanshinone IIA, a major active ingredient in the root of Salvia miltiorrhiza Bunge.Curr Drug Metab. 2007 May;8(4):325-40. doi: 10.2174/138920007780655450. Curr Drug Metab. 2007. PMID: 17504222
-
In vitro interaction of the HIV protease inhibitor ritonavir with herbal constituents: changes in P-gp and CYP3A4 activity.Am J Ther. 2004 Jul-Aug;11(4):262-77. doi: 10.1097/01.mjt.0000101827.94820.22. Am J Ther. 2004. PMID: 15266218
-
The H2 receptor antagonist nizatidine is a P-glycoprotein substrate: characterization of its intestinal epithelial cell efflux transport.AAPS J. 2009 Jun;11(2):205-13. doi: 10.1208/s12248-009-9092-5. Epub 2009 Mar 25. AAPS J. 2009. PMID: 19319690 Free PMC article.
Cited by
-
A simple medium enables bovine embryos to be held for seven days at 4°C.Sci Rep. 2013;3:1173. doi: 10.1038/srep01173. Epub 2013 Jan 30. Sci Rep. 2013. PMID: 23378907 Free PMC article.
-
Allosteric modulation of alpha4beta2 nicotinic acetylcholine receptors by HEPES.Eur J Pharmacol. 2014 Jun 5;732:159-68. doi: 10.1016/j.ejphar.2012.06.001. Epub 2012 Jun 23. Eur J Pharmacol. 2014. PMID: 22732654 Free PMC article.
-
Azetidines Kill Multidrug-Resistant Mycobacterium tuberculosis without Detectable Resistance by Blocking Mycolate Assembly.J Med Chem. 2024 Feb 22;67(4):2529-2548. doi: 10.1021/acs.jmedchem.3c01643. Epub 2024 Feb 8. J Med Chem. 2024. PMID: 38331432 Free PMC article.
-
Honokiol affects melanoma cell growth by targeting the AMP-activated protein kinase signaling pathway.Am J Surg. 2014 Dec;208(6):995-1002; discussion 1001-2. doi: 10.1016/j.amjsurg.2014.09.014. Epub 2014 Oct 2. Am J Surg. 2014. PMID: 25450590 Free PMC article.
-
Comparison of cell-based versus cell-free mammalian systems for the production of a recombinant human bone morphogenic growth factor.Eng Life Sci. 2017 Aug 7;17(10):1097-1107. doi: 10.1002/elsc.201700005. eCollection 2017 Oct. Eng Life Sci. 2017. PMID: 32624737 Free PMC article.
References
-
- Williamson JD, Cox P. Use of a new buffer in the culture of animal cells. J Gen Virol. 1968;2:309–312. - PubMed
-
- Lleu PL, Rebel G. Effect of HEPES on the taurine uptake by cultured glial cells. J Neurosci Res. 1989;23:78–86. - PubMed
-
- Rebel G, Lleu PL, Petegnief V, Frauli-Meischner M, Guerin P, Lelong IH. Effect of HEPES on the uptake of taurine by cultured nervous cells. Adv Exp Med Bio. 1992;315:277–285. - PubMed
-
- Zigger JS, Jr, Lepe-Zuniga JL, Vistica B, Gery I. Analysis of the cytotoxic effects of light-exposed HEPES–containing culture medium. In vitro Cell Dev Biol. 1985;21:282–287. - PubMed
-
- Yamamoto D, Suzuki N. Blockage of chloride channels by HEPES buffer. Proc Roy Soc London, Ser B. 1987;230:93–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

