Modulation of microvascular smooth muscle adhesion and mechanotransduction by integrin-linked kinase
- PMID: 20163538
- PMCID: PMC2923833
- DOI: 10.1111/j.1549-8719.2009.00011.x
Modulation of microvascular smooth muscle adhesion and mechanotransduction by integrin-linked kinase
Abstract
Objective: In this study, we investigated the involvement of integrin-linked kinase (ILK) in the adhesion of arteriolar vascular smooth muscle cells (VSMC) to fibronectin (FN) and in the mechano-responsiveness of VSMC focal adhesions (FA).
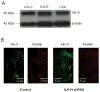
Methods: ILK was visualized in VSMC by expressing EGFP-ILK and it was knocked down using ILK-shRNA constructs. Atomic force microscopy (AFM) was used to characterize VSMC interactions with FN, VSMC stiffness and to apply and measure forces at a VSMC single FA site.
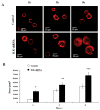
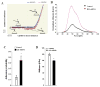
Results: ILK was localized to FA and silencing ILK promoted cell spreading, enhanced cell adhesion, reduced cell proliferation and reduced downstream phosphorylation of GSK-3beta and PKB/Akt. AFM studies demonstrated that silencing ILK enhanced alpha5beta1 integrin adhesion to FN and enhanced VSMC contraction in response to a pulling force applied at the level of a single FN-FA site.
Conclusions: ILK functions in arteriolar VSMC appear linked to multiple signaling pathways and processes that inhibit cell spreading, cell adhesion, FA formation, adhesion to FN and the mechano-responsiveness of FN-FA sites.
Figures





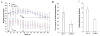
Similar articles
-
Mechanotransduction through fibronectin-integrin focal adhesion in microvascular smooth muscle cells: is calcium essential?Am J Physiol Heart Circ Physiol. 2012 May 15;302(10):H1965-73. doi: 10.1152/ajpheart.00598.2011. Epub 2012 Mar 16. Am J Physiol Heart Circ Physiol. 2012. PMID: 22427509 Free PMC article.
-
Mechanical properties of the interaction between fibronectin and alpha5beta1-integrin on vascular smooth muscle cells studied using atomic force microscopy.Am J Physiol Heart Circ Physiol. 2005 Dec;289(6):H2526-35. doi: 10.1152/ajpheart.00658.2004. Epub 2005 Aug 12. Am J Physiol Heart Circ Physiol. 2005. PMID: 16100245
-
Regulation of cell-matrix contacts and beta-catenin signaling in VSMC by integrin-linked kinase: implications for intimal thickening.Basic Res Cardiol. 2008 May;103(3):244-56. doi: 10.1007/s00395-007-0693-9. Epub 2007 Dec 13. Basic Res Cardiol. 2008. PMID: 18080083 Free PMC article.
-
Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites.Am J Physiol Cell Physiol. 2008 Jul;295(1):C268-78. doi: 10.1152/ajpcell.00516.2007. Epub 2008 May 21. Am J Physiol Cell Physiol. 2008. PMID: 18495809 Free PMC article.
-
Integrin-linked kinase (ILK): a "hot" therapeutic target.Biochem Pharmacol. 2000 Oct 15;60(8):1115-9. doi: 10.1016/s0006-2952(00)00444-5. Biochem Pharmacol. 2000. PMID: 11007949 Review.
Cited by
-
PDMS Elastic Micropost Arrays for Studying Vascular Smooth Muscle Cells.Sens Actuators B Chem. 2013 Nov 1;188:1055-1063. doi: 10.1016/j.snb.2013.08.018. Sens Actuators B Chem. 2013. PMID: 26451074 Free PMC article.
-
Effects of integrin α5β1 on the proliferation and migration of human aortic vascular smooth muscle cells.Mol Med Rep. 2016 Feb;13(2):1147-55. doi: 10.3892/mmr.2015.4649. Epub 2015 Dec 7. Mol Med Rep. 2016. PMID: 26648324 Free PMC article.
-
The Load-Bearing Mechanosome Revisited.Clin Rev Bone Miner Metab. 2010 Nov 11;8(4):213-223. doi: 10.1007/s12018-010-9075-1. Clin Rev Bone Miner Metab. 2010. PMID: 21479153 Free PMC article.
-
Formation of contractile networks and fibers in the medial cell cortex through myosin-II turnover, contraction, and stress-stabilization.Cytoskeleton (Hoboken). 2015 Jan;72(1):29-46. doi: 10.1002/cm.21207. Epub 2015 Feb 7. Cytoskeleton (Hoboken). 2015. PMID: 25641802 Free PMC article.
References
-
- Brummelkamp TR, Bernards R, et al. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296(5567):550–3. - PubMed
-
- Chao JT, Martinez-Lemus LA, et al. Modulation of alpha7-integrin-mediated adhesion and expression by platelet-derived growth factor in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2006;290(4):C972–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

