Influence of nanofibers on growth and gene expression of human tendon derived fibroblast
- PMID: 20163724
- PMCID: PMC2837661
- DOI: 10.1186/1475-925X-9-9
Influence of nanofibers on growth and gene expression of human tendon derived fibroblast
Abstract
Background: Rotator cuff tears are a common and frequent lesion especially in older patients. The mechanisms of tendon repair are not fully understood. Common therapy options for tendon repair include mini-open or arthroscopic surgery. The use of growth factors in experimental studies is mentioned in the literature. Nanofiber scaffolds, which provide several criteria for the healing process, might be a suitable therapy option for operative treatment. The aim of this study was to explore the effects of nanofiber scaffolds on human tendon derived fibroblasts (TDF's), as well as the gene expression and matrix deposition of these fibroblasts.
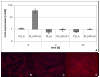
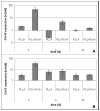
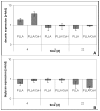
Methods: Nanofibers composed of PLLA and PLLA/Col-I were seeded with human tendon derived fibroblasts and cultivated over a period of 22 days under growth-inductive conditions, and analyzed during the course of culture, with respect to gene expression of different extra cellular matrix components such as collagens, bigylcan and decorin. Furthermore, we measured cell densities and proliferation by using fluorescence microscopy.
Results: PLLA nanofibers possessed a growth inhibitory effect on TDF's. Furthermore, no meaningful influence on the gene expression of collagen I, collagen III and decorin could be observed, while the expression of collagen X increased during the course of cultivation. On the other hand, PLLA/Col-I blend nanofibers had no negative influence on the growth of TDF's. Furthermore, blending PLLA nanofibers with collagen had a positive effect on the gene expression of collagen I, III, X and decorin. Here, gene expression indicated that focal adherence kinases might be involved.
Conclusion: This study indicates that the use of nanofibers influence expression of genes associated with the extra cellular matrix formation. The composition of the nanofibers plays a critical role. While PLLA/Col-I blend nanofibers enhance the collagen I and III formation, their expression on PLLA nanofibers was more comparable to controls. However, irrespective of the chemical composition of the fibres, the collagen deposition was altered, an effect which might be associated with a decreased expression of biglycanes.
Figures



Similar articles
-
Functionalisation of PLLA nanofiber scaffolds using a possible cooperative effect between collagen type I and BMP-2: impact on growth and osteogenic differentiation of human mesenchymal stem cells.J Mater Sci Mater Med. 2011 Jul;22(7):1753-62. doi: 10.1007/s10856-011-4341-4. Epub 2011 May 22. J Mater Sci Mater Med. 2011. PMID: 21604139 Free PMC article.
-
Influence of poly(L-lactic acid) nanofibers and BMP-2-containing poly(L-lactic acid) nanofibers on growth and osteogenic differentiation of human mesenchymal stem cells.ScientificWorldJournal. 2008 Dec 25;8:1269-79. doi: 10.1100/tsw.2008.163. ScientificWorldJournal. 2008. PMID: 19112539 Free PMC article.
-
Influence of nanofibers on the growth and osteogenic differentiation of stem cells: a comparison of biological collagen nanofibers and synthetic PLLA fibers.J Mater Sci Mater Med. 2009 Mar;20(3):767-74. doi: 10.1007/s10856-008-3634-8. Epub 2008 Nov 6. J Mater Sci Mater Med. 2009. PMID: 18987945
-
The influence of type-I collagen-coated PLLA aligned nanofibers on growth of blood outgrowth endothelial cells.Biomed Mater. 2010 Dec;5(6):065011. doi: 10.1088/1748-6041/5/6/065011. Epub 2010 Nov 9. Biomed Mater. 2010. PMID: 21060144
-
The role of injection collagen therapy in greater trochanter pain syndrome. A new therapeutic approach?Reumatologia. 2025 Apr 1;63(2):131-137. doi: 10.5114/reum/196810. eCollection 2025. Reumatologia. 2025. PMID: 40485944 Free PMC article. Review.
Cited by
-
Nanofiber Technology for Regenerative Engineering.ACS Nano. 2020 Aug 25;14(8):9347-9363. doi: 10.1021/acsnano.0c03981. Epub 2020 Jul 22. ACS Nano. 2020. PMID: 32678581 Free PMC article.
-
Tendon Fascicle-Inspired Nanofibrous Scaffold of Polylactic acid/Collagen with Enhanced 3D-Structure and Biomechanical Properties.Sci Rep. 2018 Nov 21;8(1):17167. doi: 10.1038/s41598-018-35536-8. Sci Rep. 2018. PMID: 30464300 Free PMC article.
-
Advances in Stem Cell Therapies for Rotator Cuff Injuries.Front Bioeng Biotechnol. 2022 May 25;10:866195. doi: 10.3389/fbioe.2022.866195. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35694228 Free PMC article. Review.
-
Synthetic scaffolds for musculoskeletal tissue engineering: cellular responses to fiber parameters.NPJ Regen Med. 2019 Jun 27;4:15. doi: 10.1038/s41536-019-0076-5. eCollection 2019. NPJ Regen Med. 2019. PMID: 31263573 Free PMC article. Review.
-
In vitro changes in human tenocyte cultures obtained from proximal biceps tendon: multiple passages result in changes in routine cell markers.Knee Surg Sports Traumatol Arthrosc. 2012 Sep;20(9):1666-72. doi: 10.1007/s00167-011-1711-x. Epub 2011 Oct 18. Knee Surg Sports Traumatol Arthrosc. 2012. PMID: 22005966
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

