Mild hypothermia alters midazolam pharmacokinetics in normal healthy volunteers
- PMID: 20164112
- PMCID: PMC2872942
- DOI: 10.1124/dmd.109.031377
Mild hypothermia alters midazolam pharmacokinetics in normal healthy volunteers
Abstract
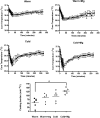
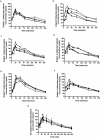
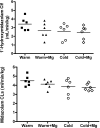
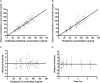
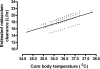
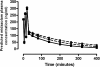
The clinical use of therapeutic hypothermia has been rapidly expanding due to evidence of neuroprotection. However, the effect of hypothermia on specific pathways of drug elimination in humans is relatively unknown. To gain insight into the potential effects of hypothermia on drug metabolism and disposition, we evaluated the pharmacokinetics of midazolam as a probe for CYP3A4/5 activity during mild hypothermia in human volunteers. A second objective of this work was to determine whether benzodiazepines and magnesium administered intravenously would facilitate the induction of hypothermia. Subjects were enrolled in a randomized crossover study, which included two mild hypothermia groups (4 degrees C saline infusions and 4 degrees C saline + magnesium) and two normothermia groups (37 degrees C saline infusions and 37 degrees C saline + magnesium). The lowest temperatures achieved in the 4 degrees C saline + magnesium and 4 degrees C saline infusions were 35.4 +/- 0.4 and 35.8 +/- 0.3 degrees C, respectively. A significant decrease in the formation clearance of the major metabolite 1'-hydroxymidazolam was observed during the 4 degrees C saline + magnesium compared with that in the 37 degrees C saline group (p < 0.05). Population pharmacokinetic modeling identified a significant relationship between temperature and clearance and intercompartmental clearance for midazolam. This model predicted that midazolam clearance decreases 11.1% for each degree Celsius reduction in core temperature from 36.5 degrees C. Midazolam with magnesium facilitated the induction of hypothermia, but shivering was minimally suppressed. These data provided proof of concept that even mild and short-duration changes in body temperature significantly affect midazolam metabolism. Future studies in patients who receive lower levels and a longer duration of hypothermia are warranted.
Figures
Similar articles
-
The effect of multiple-dose, oral rifaximin on the pharmacokinetics of intravenous and oral midazolam in healthy volunteers.Pharmacotherapy. 2007 Oct;27(10):1361-9. doi: 10.1592/phco.27.10.1361. Pharmacotherapy. 2007. PMID: 17896891 Clinical Trial.
-
Mild hypothermia decreases fentanyl and midazolam steady-state clearance in a rat model of cardiac arrest.Crit Care Med. 2012 Apr;40(4):1221-8. doi: 10.1097/CCM.0b013e31823779f9. Crit Care Med. 2012. PMID: 22067624 Free PMC article.
-
High-dose diazepam facilitates core cooling during cold saline infusion in healthy volunteers.Appl Physiol Nutr Metab. 2009 Aug;34(4):582-6. doi: 10.1139/H09-011. Appl Physiol Nutr Metab. 2009. PMID: 19767791 Clinical Trial.
-
Impact of the Selective Orexin-1 Receptor Antagonist ACT-539313 on the Pharmacokinetics of the CYP3A Probe Drug Midazolam in Healthy Male Subjects.J Clin Pharmacol. 2020 Jul;60(7):931-941. doi: 10.1002/jcph.1588. Epub 2020 Feb 8. J Clin Pharmacol. 2020. PMID: 32035014 Clinical Trial.
-
The effect of therapeutic hypothermia on drug metabolism and response: cellular mechanisms to organ function.Expert Opin Drug Metab Toxicol. 2011 Jul;7(7):803-16. doi: 10.1517/17425255.2011.574127. Epub 2011 Apr 8. Expert Opin Drug Metab Toxicol. 2011. PMID: 21473710 Free PMC article. Review.
Cited by
-
Pharmacokinetics and pharmacodynamics of medication in asphyxiated newborns during controlled hypothermia. The PharmaCool multicenter study.BMC Pediatr. 2012 May 22;12:45. doi: 10.1186/1471-2431-12-45. BMC Pediatr. 2012. PMID: 22515424 Free PMC article.
-
Effects of Propofol on Hemodynamic Profile in Adults Receiving Targeted Temperature Management.Hosp Pharm. 2022 Jun;57(3):329-335. doi: 10.1177/00185787211032359. Epub 2021 Jul 11. Hosp Pharm. 2022. PMID: 35615480 Free PMC article.
-
A Physiology-Based Pharmacokinetic Framework to Support Drug Development and Dose Precision During Therapeutic Hypothermia in Neonates.Front Pharmacol. 2020 May 13;11:587. doi: 10.3389/fphar.2020.00587. eCollection 2020. Front Pharmacol. 2020. PMID: 32477113 Free PMC article. Review.
-
Temperature control after cardiac arrest.Resuscitation. 2023 Aug;189:109882. doi: 10.1016/j.resuscitation.2023.109882. Epub 2023 Jun 23. Resuscitation. 2023. PMID: 37355091 Free PMC article. Review.
-
State-of-the-art considerations in post-arrest care.J Am Coll Emerg Physicians Open. 2020 Mar 8;1(2):107-116. doi: 10.1002/emp2.12022. eCollection 2020 Apr. J Am Coll Emerg Physicians Open. 2020. PMID: 33000021 Free PMC article. Review.
References
-
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. (2002) Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346:557–563 - PubMed
-
- Bolon M, Bastien O, Flamens C, Paulus S, Salord F, Boulieu R. (2003) Evaluation of the estimation of midazolam concentrations and pharmacokinetic parameters in intensive care patients using a bayesian pharmacokinetic software (PKS) according to sparse sampling approach. J Pharm Pharmacol 55:765–771 - PubMed
-
- Caldwell JE, Heier T, Wright PM, Lin S, McCarthy G, Szenohradszky J, Sharma ML, Hing JP, Schroeder M, Sessler DI. (2000) Temperature-dependent pharmacokinetics and pharmacodynamics of vecuronium. Anesthesiology 92:84–93 - PubMed
-
- de Wildt SN, de Hoog M, Vinks AA, van der Giesen E, van den Anker JN. (2003) Population pharmacokinetics and metabolism of midazolam in pediatric intensive care patients. Crit Care Med 31:1952–1958 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

