NF-kappaB activation in T cells requires discrete control of IkappaB kinase alpha/beta (IKKalpha/beta) phosphorylation and IKKgamma ubiquitination by the ADAP adapter protein
- PMID: 20164171
- PMCID: PMC2856986
- DOI: 10.1074/jbc.M109.068999
NF-kappaB activation in T cells requires discrete control of IkappaB kinase alpha/beta (IKKalpha/beta) phosphorylation and IKKgamma ubiquitination by the ADAP adapter protein
Abstract
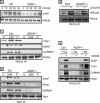
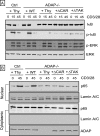
NF-kappaB activation following engagement of the antigen-specific T cell receptor involves protein kinase C-theta-dependent assembly of the CARMA1-BCL10-MALT1 (CBM) signalosome, which coordinates downstream activation of IkappaB kinase (IKK). We previously identified a novel role for the adhesion- and degranulation-promoting adapter protein (ADAP) in regulating the assembly of the CBM complex via an interaction of ADAP with CARMA1. In this study, we identify a novel site in ADAP that is critical for association with the TAK1 kinase. ADAP is critical for recruitment of TAK1 and the CBM complex, but not IKK, to protein kinase C-theta. ADAP is not required for TAK1 activation. Although both the TAK1 and the CARMA1 binding sites in ADAP are essential for IkappaB alpha phosphorylation and degradation and NF-kappaB nuclear translocation, only the TAK1 binding site in ADAP is necessary for IKK phosphorylation. In contrast, only the CARMA1 binding site in ADAP is required for ubiquitination of IKKgamma. Thus, distinct sites within ADAP control two key activation responses that are required for NF-kappaB activation in T cells.
Figures


Similar articles
-
ADAP regulates cell cycle progression of T cells via control of cyclin E and Cdk2 expression through two distinct CARMA1-dependent signaling pathways.Mol Cell Biol. 2012 May;32(10):1908-17. doi: 10.1128/MCB.06541-11. Epub 2012 Mar 12. Mol Cell Biol. 2012. PMID: 22411628 Free PMC article.
-
Regulation of NF-kappaB activation in T cells via association of the adapter proteins ADAP and CARMA1.Science. 2007 May 4;316(5825):754-8. doi: 10.1126/science.1137895. Science. 2007. PMID: 17478723
-
The pleckstrin homology domain in the SKAP55 adapter protein defines the ability of the adapter protein ADAP to regulate integrin function and NF-kappaB activation.J Immunol. 2011 Jun 1;186(11):6227-37. doi: 10.4049/jimmunol.1002950. Epub 2011 Apr 27. J Immunol. 2011. PMID: 21525391 Free PMC article.
-
TCR signaling to NF-κB and mTORC1: Expanding roles of the CARMA1 complex.Mol Immunol. 2015 Dec;68(2 Pt C):546-57. doi: 10.1016/j.molimm.2015.07.024. Epub 2015 Aug 8. Mol Immunol. 2015. PMID: 26260210 Free PMC article. Review.
-
Role of the CARMA1/BCL10/MALT1 complex in lymphoid malignancies.Curr Opin Hematol. 2016 Jul;23(4):402-9. doi: 10.1097/MOH.0000000000000257. Curr Opin Hematol. 2016. PMID: 27135977 Free PMC article. Review.
Cited by
-
The Multiple Roles of the Cytosolic Adapter Proteins ADAP, SKAP1 and SKAP2 for TCR/CD3 -Mediated Signaling Events.Front Immunol. 2021 Jul 6;12:703534. doi: 10.3389/fimmu.2021.703534. eCollection 2021. Front Immunol. 2021. PMID: 34295339 Free PMC article. Review.
-
Implications of a 'Third Signal' in NK Cells.Cells. 2021 Jul 31;10(8):1955. doi: 10.3390/cells10081955. Cells. 2021. PMID: 34440725 Free PMC article. Review.
-
Multistage T cell-dendritic cell interactions control optimal CD4 T cell activation through the ADAP-SKAP55-signaling module.J Immunol. 2013 Sep 1;191(5):2372-83. doi: 10.4049/jimmunol.1300107. Epub 2013 Aug 5. J Immunol. 2013. PMID: 23918975 Free PMC article.
-
Controlling Cytokine Release Syndrome to Harness the Full Potential of CAR-Based Cellular Therapy.Front Oncol. 2020 Jan 31;9:1529. doi: 10.3389/fonc.2019.01529. eCollection 2019. Front Oncol. 2020. PMID: 32076597 Free PMC article. Review.
-
T-cell receptor-induced JNK activation requires proteolytic inactivation of CYLD by MALT1.EMBO J. 2011 May 4;30(9):1742-52. doi: 10.1038/emboj.2011.85. Epub 2011 Mar 29. EMBO J. 2011. PMID: 21448133 Free PMC article.
References
-
- Vallabhapurapu S., Karin M. (2009) Annu. Rev. Immunol. 27, 693–733 - PubMed
-
- Hayden M. S., West A. P., Ghosh S. (2006) Oncogene 25, 6758–6780 - PubMed
-
- Khoshnan A., Bae D., Tindell C. A., Nel A. E. (2000) J. Immunol. 165, 6933–6940 - PubMed
-
- Matsumoto R., Wang D., Blonska M., Li H., Kobayashi M., Pappu B., Chen Y., Wang D., Lin X. (2005) Immunity 23, 575–585 - PubMed
-
- Sommer K., Guo B., Pomerantz J. L., Bandaranayake A. D., Moreno-García M. E., Ovechkina Y. L., Rawlings D. J. (2005) Immunity 23, 561–574 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

