Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65
- PMID: 20164172
- PMCID: PMC3282996
- DOI: 10.1074/jbc.M109.099630
Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65
Abstract
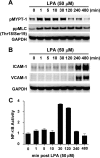
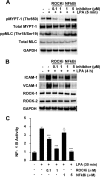
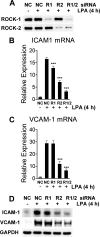
Endothelial cells play an important role in the recruitment of immune cells to a disease locus through the induced expression of chemokines and cell adhesion molecules (CAMs). The proinflammatory lysophospholipid, lysophosphatidic acid (LPA), which is elevated in multiple inflammatory diseases, is a potent activator of the RhoA/Rho kinase signaling pathway and has been shown to induce the expression of CAMs in endothelial cells. The present study was undertaken to map signal transduction downstream of LPA and to investigate the contributions of the Rho kinase isoforms ROCK1 and ROCK2 to adhesion molecule expression in human umbilical vein endothelial cells. LPA activated Rho kinase within minutes and subsequently the NF-kappaB pathway through phosphorylation of the p65 subunit. The lipid also induced the late expression of intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). Pharmacologic inhibition of Rho kinase signaling blocked LPA-induced p65 phosphorylation and suppressed ICAM-1 and VCAM-1 expression. Inhibition of the NF-kappaB pathway had no impact on LPA-induced Rho kinase activation, but inhibited adhesion molecule expression. Small interfering RNA-facilitated knockdown of each isoform identified ROCK2 as the mediator of LPA-driven phosphorylation of NF-kappaB p65 and of ICAM-1 and VCAM-1 mRNA and protein induction. Taken collectively, our data are consistent with Rho kinase being upstream of NF-kappaB in driving LPA-mediated adhesion molecule expression. This study also provides the first evidence of the critical involvement of ROCK2 in LPA-induced CAM expression through activation of the NF-kappaB pathway in human endothelial cells.
Figures


Similar articles
-
ROCK2 Regulates Monocyte Migration and Cell to Cell Adhesion in Vascular Endothelial Cells.Int J Mol Sci. 2019 Mar 16;20(6):1331. doi: 10.3390/ijms20061331. Int J Mol Sci. 2019. PMID: 30884801 Free PMC article.
-
RhoA/Rho-associated kinase pathway selectively regulates thrombin-induced intercellular adhesion molecule-1 expression in endothelial cells via activation of I kappa B kinase beta and phosphorylation of RelA/p65.J Immunol. 2004 Dec 1;173(11):6965-72. doi: 10.4049/jimmunol.173.11.6965. J Immunol. 2004. PMID: 15557193
-
Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells.J Biol Chem. 2001 Mar 9;276(10):7614-20. doi: 10.1074/jbc.M009705200. Epub 2000 Dec 6. J Biol Chem. 2001. PMID: 11108718
-
Lysophosphatidic Acid Regulates Rho Family of GTPases in Lungs.Cell Biochem Biophys. 2021 Sep;79(3):493-496. doi: 10.1007/s12013-021-00993-y. Epub 2021 Jun 10. Cell Biochem Biophys. 2021. PMID: 34110567 Review.
-
Regulation of angiogenesis by phospholipid lysophosphatidic acid.Front Biosci (Landmark Ed). 2013 Jun 1;18(3):852-61. doi: 10.2741/4148. Front Biosci (Landmark Ed). 2013. PMID: 23747852 Review.
Cited by
-
Role of ROCK/NF‑κB/AQP8 signaling in ethanol‑induced intestinal epithelial barrier dysfunction.Mol Med Rep. 2020 Sep;22(3):2253-2262. doi: 10.3892/mmr.2020.11318. Epub 2020 Jul 9. Mol Med Rep. 2020. PMID: 32705263 Free PMC article.
-
Deregulated Lysophosphatidic Acid Metabolism and Signaling in Liver Cancer.Cancers (Basel). 2019 Oct 23;11(11):1626. doi: 10.3390/cancers11111626. Cancers (Basel). 2019. PMID: 31652837 Free PMC article. Review.
-
Inhibiting NF-κB activation by small molecules as a therapeutic strategy.Biochim Biophys Acta. 2010 Oct-Dec;1799(10-12):775-87. doi: 10.1016/j.bbagrm.2010.05.004. Epub 2010 May 21. Biochim Biophys Acta. 2010. PMID: 20493977 Free PMC article. Review.
-
ROCK2 Regulates Monocyte Migration and Cell to Cell Adhesion in Vascular Endothelial Cells.Int J Mol Sci. 2019 Mar 16;20(6):1331. doi: 10.3390/ijms20061331. Int J Mol Sci. 2019. PMID: 30884801 Free PMC article.
-
The role of RhoA/Rho kinase pathway in endothelial dysfunction.J Cardiovasc Dis Res. 2010 Oct;1(4):165-70. doi: 10.4103/0975-3583.74258. J Cardiovasc Dis Res. 2010. PMID: 21264179 Free PMC article.
References
-
- Marlin S. D., Springer T. A. (1987) Cell 51, 813–819 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

