Reaction mechanism and molecular basis for selenium/sulfur discrimination of selenocysteine lyase
- PMID: 20164179
- PMCID: PMC2852952
- DOI: 10.1074/jbc.M109.084475
Reaction mechanism and molecular basis for selenium/sulfur discrimination of selenocysteine lyase
Abstract
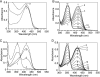
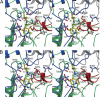
Selenocysteine lyase (SCL) catalyzes the pyridoxal 5'-phosphate-dependent removal of selenium from l-selenocysteine to yield l-alanine. The enzyme is proposed to function in the recycling of the micronutrient selenium from degraded selenoproteins containing selenocysteine residue as an essential component. The enzyme exhibits strict substrate specificity toward l-selenocysteine and no activity to its cognate l-cysteine. However, it remains unclear how the enzyme distinguishes between selenocysteine and cysteine. Here, we present mechanistic studies of selenocysteine lyase from rat. ESI-MS analysis of wild-type and C375A mutant SCL revealed that the catalytic reaction proceeds via the formation of an enzyme-bound selenopersulfide intermediate on the catalytically essential Cys-375 residue. UV-visible spectrum analysis and the crystal structure of SCL complexed with l-cysteine demonstrated that the enzyme reversibly forms a nonproductive adduct with l-cysteine. Cys-375 on the flexible loop directed l-selenocysteine, but not l-cysteine, to the correct position and orientation in the active site to initiate the catalytic reaction. These findings provide, for the first time, the basis for understanding how trace amounts of a selenium-containing substrate is distinguished from excessive amounts of its cognate sulfur-containing compound in a biological system.
Figures

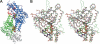
Similar articles
-
Biochemical discrimination between selenium and sulfur 2: mechanistic investigation of the selenium specificity of human selenocysteine lyase.PLoS One. 2012;7(1):e30528. doi: 10.1371/journal.pone.0030528. Epub 2012 Jan 26. PLoS One. 2012. PMID: 22291978 Free PMC article.
-
Biochemical discrimination between selenium and sulfur 1: a single residue provides selenium specificity to human selenocysteine lyase.PLoS One. 2012;7(1):e30581. doi: 10.1371/journal.pone.0030581. Epub 2012 Jan 25. PLoS One. 2012. PMID: 22295093 Free PMC article.
-
Structure of external aldimine of Escherichia coli CsdB, an IscS/NifS homolog: implications for its specificity toward selenocysteine.J Biochem. 2002 May;131(5):679-85. doi: 10.1093/oxfordjournals.jbchem.a003151. J Biochem. 2002. PMID: 11983074
-
Utilization of selenocysteine as a source of selenium for selenophosphate biosynthesis.Biofactors. 2001;14(1-4):69-74. doi: 10.1002/biof.5520140110. Biofactors. 2001. PMID: 11568442 Review.
-
Cysteine S-conjugate β-lyases: important roles in the metabolism of naturally occurring sulfur and selenium-containing compounds, xenobiotics and anticancer agents.Amino Acids. 2011 Jun;41(1):7-27. doi: 10.1007/s00726-010-0552-0. Epub 2010 Mar 22. Amino Acids. 2011. PMID: 20306345 Free PMC article. Review.
Cited by
-
Structural changes during cysteine desulfurase CsdA and sulfur acceptor CsdE interactions provide insight into the trans-persulfuration.J Biol Chem. 2013 Sep 20;288(38):27172-27180. doi: 10.1074/jbc.M113.480277. Epub 2013 Aug 2. J Biol Chem. 2013. PMID: 23913692 Free PMC article.
-
A selenium-dependent xanthine dehydrogenase triggers biofilm proliferation in Enterococcus faecalis through oxidant production.J Bacteriol. 2011 Apr;193(7):1643-52. doi: 10.1128/JB.01063-10. Epub 2011 Jan 21. J Bacteriol. 2011. PMID: 21257770 Free PMC article.
-
Trypanosomatid selenophosphate synthetase structure, function and interaction with selenocysteine lyase.PLoS Negl Trop Dis. 2020 Oct 5;14(10):e0008091. doi: 10.1371/journal.pntd.0008091. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33017394 Free PMC article.
-
Relationship between selenoprotein P and selenocysteine lyase: Insights into selenium metabolism.Free Radic Biol Med. 2018 Nov 1;127:182-189. doi: 10.1016/j.freeradbiomed.2018.03.037. Epub 2018 Mar 20. Free Radic Biol Med. 2018. PMID: 29567390 Free PMC article.
-
Role of selenium in the pathophysiology of cardiorenal anaemia syndrome.ESC Heart Fail. 2025 Apr;12(2):770-780. doi: 10.1002/ehf2.14893. Epub 2024 Sep 2. ESC Heart Fail. 2025. PMID: 39223820 Free PMC article. Review.
References
-
- Hatfield D. L. (2001) Selenium: Its Molecular Biology and Role in Human Health, Kluwer Academic Publishers, Norwell, MA
-
- Small-Howard A. L., Berry M. J. (2005) Biochem. Soc. Trans. 33, 1493–1497 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

