Reaction mechanism and molecular basis for selenium/sulfur discrimination of selenocysteine lyase
- PMID: 20164179
- PMCID: PMC2852952
- DOI: 10.1074/jbc.M109.084475
Reaction mechanism and molecular basis for selenium/sulfur discrimination of selenocysteine lyase
Abstract
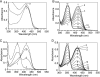
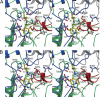
Selenocysteine lyase (SCL) catalyzes the pyridoxal 5'-phosphate-dependent removal of selenium from l-selenocysteine to yield l-alanine. The enzyme is proposed to function in the recycling of the micronutrient selenium from degraded selenoproteins containing selenocysteine residue as an essential component. The enzyme exhibits strict substrate specificity toward l-selenocysteine and no activity to its cognate l-cysteine. However, it remains unclear how the enzyme distinguishes between selenocysteine and cysteine. Here, we present mechanistic studies of selenocysteine lyase from rat. ESI-MS analysis of wild-type and C375A mutant SCL revealed that the catalytic reaction proceeds via the formation of an enzyme-bound selenopersulfide intermediate on the catalytically essential Cys-375 residue. UV-visible spectrum analysis and the crystal structure of SCL complexed with l-cysteine demonstrated that the enzyme reversibly forms a nonproductive adduct with l-cysteine. Cys-375 on the flexible loop directed l-selenocysteine, but not l-cysteine, to the correct position and orientation in the active site to initiate the catalytic reaction. These findings provide, for the first time, the basis for understanding how trace amounts of a selenium-containing substrate is distinguished from excessive amounts of its cognate sulfur-containing compound in a biological system.
Figures

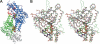
References
-
- Hatfield D. L. (2001) Selenium: Its Molecular Biology and Role in Human Health, Kluwer Academic Publishers, Norwell, MA
-
- Small-Howard A. L., Berry M. J. (2005) Biochem. Soc. Trans. 33, 1493–1497 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

