A novel LZAP-binding protein, NLBP, inhibits cell invasion
- PMID: 20164180
- PMCID: PMC2852962
- DOI: 10.1074/jbc.M109.065920
A novel LZAP-binding protein, NLBP, inhibits cell invasion
Abstract
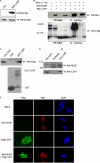
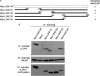
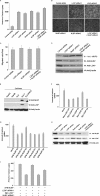
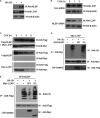
LXXLL/leucine zipper-containing alternative reading frame (ARF)-binding protein (LZAP) was recently shown to function as a tumor suppressor through inhibition of the NF-kappaB signaling pathway. LZAP is also known as a negative regulator of cell invasion, and its expression was demonstrated to be reduced in several tumor tissues. However, the molecular mechanism of the negative effect of LZAP on cell invasion is unclear. In this study, we identify NLBP as a novel LZAP-binding protein using tandem affinity purification. We demonstrate the negative effects of NLBP on cell invasion and the NF-kappaB signaling pathway. NLBP expression was not detected in hepatocellular carcinoma cells with strong invasive activity, whereas its expression was detected in a hepatocellular carcinoma cell line with no invasive activity. We also demonstrate that these two proteins mutually affect the stability of each other by inhibiting ubiquitination of the other protein. Based on these results, we suggest that NLBP may act as a novel tumor suppressor by inhibiting cell invasion, blocking NF-kappaB signaling, and increasing stability of the LZAP protein.
Figures


Similar articles
-
A novel ARF-binding protein (LZAP) alters ARF regulation of HDM2.Biochem J. 2006 Jan 15;393(Pt 2):489-501. doi: 10.1042/BJ20050960. Biochem J. 2006. PMID: 16173922 Free PMC article.
-
A novel C53/LZAP-interacting protein regulates stability of C53/LZAP and DDRGK domain-containing Protein 1 (DDRGK1) and modulates NF-kappaB signaling.J Biol Chem. 2010 May 14;285(20):15126-15136. doi: 10.1074/jbc.M110.110619. Epub 2010 Mar 12. J Biol Chem. 2010. PMID: 20228063 Free PMC article.
-
LZAP, a putative tumor suppressor, selectively inhibits NF-kappaB.Cancer Cell. 2007 Sep;12(3):239-51. doi: 10.1016/j.ccr.2007.07.002. Cancer Cell. 2007. PMID: 17785205
-
Caspase-mediated cleavage of C53/LZAP protein causes abnormal microtubule bundling and rupture of the nuclear envelope.Cell Res. 2013 May;23(5):691-704. doi: 10.1038/cr.2013.36. Epub 2013 Mar 12. Cell Res. 2013. PMID: 23478299 Free PMC article.
-
Negative regulation of RelA phosphorylation: emerging players and their roles in cancer.Cytokine Growth Factor Rev. 2015 Feb;26(1):7-13. doi: 10.1016/j.cytogfr.2014.09.003. Epub 2014 Oct 2. Cytokine Growth Factor Rev. 2015. PMID: 25438737 Review.
Cited by
-
Ufm1-Specific Ligase Ufl1 Regulates Endoplasmic Reticulum Homeostasis and Protects Against Heart Failure.Circ Heart Fail. 2018 Oct;11(10):e004917. doi: 10.1161/CIRCHEARTFAILURE.118.004917. Circ Heart Fail. 2018. PMID: 30354401 Free PMC article.
-
CDK5RAP3 suppresses Wnt/β-catenin signaling by inhibiting AKT phosphorylation in gastric cancer.J Exp Clin Cancer Res. 2018 Mar 14;37(1):59. doi: 10.1186/s13046-018-0716-4. J Exp Clin Cancer Res. 2018. PMID: 29540196 Free PMC article.
-
UFBP1, a Key Component of the Ufm1 Conjugation System, Is Essential for Ufmylation-Mediated Regulation of Erythroid Development.PLoS Genet. 2015 Nov 6;11(11):e1005643. doi: 10.1371/journal.pgen.1005643. eCollection 2015 Nov. PLoS Genet. 2015. PMID: 26544067 Free PMC article.
-
Robust gene expression changes in the ganglia following subclinical reactivation in rhesus macaques infected with simian varicella virus.J Neurovirol. 2017 Aug;23(4):520-538. doi: 10.1007/s13365-017-0522-3. Epub 2017 Mar 20. J Neurovirol. 2017. PMID: 28321697 Free PMC article.
-
A critical role of DDRGK1 in endoplasmic reticulum homoeostasis via regulation of IRE1α stability.Nat Commun. 2017 Jan 27;8:14186. doi: 10.1038/ncomms14186. Nat Commun. 2017. PMID: 28128204 Free PMC article.
References
-
- Hahn W. C., Weinberg R. A. (2002) N. Engl. J. Med. 347, 1593–1603 - PubMed
-
- Wiedemann L. M., Morgan G. J. (1992) Eur. J. Cancer 28, 248–251 - PubMed
-
- Jiang H., Luo S., Li H. (2005) J. Biol. Chem. 280, 20651–20659 - PubMed
-
- Wang X., Ching Y. P., Lam W. H., Qi Z., Zhang M., Wang J. H. (2000) J. Biol. Chem. 275, 31763–31769 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

