Critical role of the platelet-derived growth factor receptor (PDGFR) beta transmembrane domain in the TEL-PDGFRbeta cytosolic oncoprotein
- PMID: 20164181
- PMCID: PMC2852966
- DOI: 10.1074/jbc.M109.076638
Critical role of the platelet-derived growth factor receptor (PDGFR) beta transmembrane domain in the TEL-PDGFRbeta cytosolic oncoprotein
Abstract
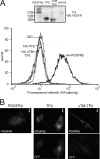
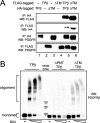
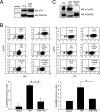
The fusion of TEL with platelet-derived growth factor receptor (PDGFR) beta (TPbeta) is found in a subset of patients with atypical myeloid neoplasms associated with eosinophilia and is the archetype of a larger group of hybrid receptors that are produced by rearrangements of PDGFR genes. TPbeta is activated by oligomerization mediated by the pointed domain of TEL/ETV6, leading to constitutive activation of the PDGFRbeta kinase domain. The receptor transmembrane (TM) domain is retained in TPbeta and in most of the described PDGFRbeta hybrids. Deletion of the TM domain (DeltaTM-TPbeta) strongly impaired the ability of TPbeta to sustain growth factor-independent cell proliferation. We confirmed that TPbeta resides in the cytosol, indicating that the PDGFRbeta TM domain does not act as a transmembrane domain in the context of the hybrid receptor but has a completely different function. The DeltaTM-TPbeta protein was expressed at a lower level because of increased degradation. It could form oligomers, was phosphorylated at a slightly higher level, co-immunoprecipitated with the p85 adaptor protein, but showed a much reduced capacity to activate STAT5 and ERK1/2 in Ba/F3 cells, compared with TPbeta. In an in vitro kinase assay, DeltaTM-TPbeta was more active than TPbeta and less sensitive to imatinib, a PDGFR inhibitor. In conclusion, we show that the TM domain is required for TPbeta-mediated signaling and proliferation, suggesting that the activation of the PDGFRbeta kinase domain is not enough for cell transformation.
Figures





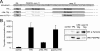
Similar articles
-
The fusion proteins TEL-PDGFRbeta and FIP1L1-PDGFRalpha escape ubiquitination and degradation.Haematologica. 2009 Aug;94(8):1085-93. doi: 10.3324/haematol.2008.001149. Haematologica. 2009. PMID: 19644140 Free PMC article.
-
Multiple oligomerization domains of KANK1-PDGFRβ are required for JAK2-independent hematopoietic cell proliferation and signaling via STAT5 and ERK.Haematologica. 2011 Oct;96(10):1406-14. doi: 10.3324/haematol.2011.040147. Epub 2011 Jun 17. Haematologica. 2011. PMID: 21685469 Free PMC article.
-
Tel/PDGFRbeta induces stem cell differentiation via the Ras/ERK and STAT5 signaling pathways.Exp Hematol. 2009 Jan;37(1):111-121. doi: 10.1016/j.exphem.2008.09.012. Exp Hematol. 2009. PMID: 19100521
-
ETV6-NTRK3: a chimeric protein tyrosine kinase with transformation activity in multiple cell lineages.Semin Cancer Biol. 2005 Jun;15(3):215-23. doi: 10.1016/j.semcancer.2005.01.003. Semin Cancer Biol. 2005. PMID: 15826836 Review.
-
Platelet-derived growth factor receptors (PDGFRs) fusion genes involvement in hematological malignancies.Crit Rev Oncol Hematol. 2017 Jan;109:20-34. doi: 10.1016/j.critrevonc.2016.11.008. Epub 2016 Nov 21. Crit Rev Oncol Hematol. 2017. PMID: 28010895 Review.
Cited by
-
Platelet-derived growth factors and their receptors in normal and malignant hematopoiesis.Am J Blood Res. 2012;2(1):44-56. Epub 2012 Jan 1. Am J Blood Res. 2012. PMID: 22432087 Free PMC article.
-
Targeting the PDGF signaling pathway in tumor treatment.Cell Commun Signal. 2013 Dec 20;11:97. doi: 10.1186/1478-811X-11-97. Cell Commun Signal. 2013. PMID: 24359404 Free PMC article. Review.
-
Molecular Pathogenesis and Treatment Perspectives for Hypereosinophilia and Hypereosinophilic Syndromes.Int J Mol Sci. 2021 Jan 6;22(2):486. doi: 10.3390/ijms22020486. Int J Mol Sci. 2021. PMID: 33418988 Free PMC article. Review.
-
Autocrine PDGF stimulation in malignancies.Ups J Med Sci. 2012 May;117(2):83-91. doi: 10.3109/03009734.2012.658119. Ups J Med Sci. 2012. PMID: 22512244 Free PMC article. Review.
-
Tyrosine kinase gene fusions in cancer: translating mechanisms into targeted therapies.J Cell Mol Med. 2012 Feb;16(2):237-48. doi: 10.1111/j.1582-4934.2011.01415.x. J Cell Mol Med. 2012. PMID: 21854543 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

