Hepatitis B virus protein X-induced expression of the CXC chemokine IP-10 is mediated through activation of NF-kappaB and increases migration of leukocytes
- PMID: 20164184
- PMCID: PMC2852955
- DOI: 10.1074/jbc.M109.067629
Hepatitis B virus protein X-induced expression of the CXC chemokine IP-10 is mediated through activation of NF-kappaB and increases migration of leukocytes
Abstract
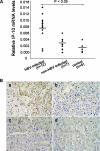
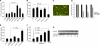
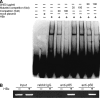
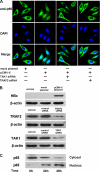
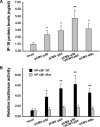
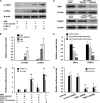
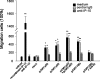
Interferon-gamma inducible protein 10 (IP-10) involves inflammatory cell recruitment and cellular immune damage during virus infection. Although an increase of the peripheral IP-10 level is known in HBV-infected patients, the molecular basis of HBV infection inducing IP-10 expression has remained elusive. In the present study, we demonstrate that hepatitis B virus protein X (HBx) increases IP-10 expression in a dose-dependent manner. Transfection of the HBx-expressing vector into HepG2 cells results in nuclear translocation of NF-kappaB, which directly binds the promoter of IP-10 at positions from -122 to -113, thus facilitating transcription. The addition of the NF-kappaB inhibitor blocks the effect of HBx on IP-10 induction. In parallel, increase of NF-kappaB subunits p65 and p50 in HepG2 cells also augments IP-10 expression. Furthermore, we show that HBx induces activation of NF-kappaB through the TRAF2/TAK1 signaling pathway, leading to up-regulation of IP-10 expression. As a consequence, up-regulation of IP-10 may mediate the migration of peripheral blood leukocytes in a NF-kappaB-dependent manner. In conclusion, we report a novel molecular mechanism of HBV infection inducing IP-10 expression, which involves viral protein HBx affecting NF-kappaB pathway, leading to transactivation of the IP-10 promoter. Our study provides insight into the migration of leukocytes in response to HBV infection, thus causing immune pathological injury of liver.
Figures

Similar articles
-
RPS3a over-expressed in HBV-associated hepatocellular carcinoma enhances the HBx-induced NF-κB signaling via its novel chaperoning function.PLoS One. 2011;6(8):e22258. doi: 10.1371/journal.pone.0022258. Epub 2011 Aug 16. PLoS One. 2011. PMID: 21857917 Free PMC article.
-
Upregulation of IL-23 expression in patients with chronic hepatitis B is mediated by the HBx/ERK/NF-κB pathway.J Immunol. 2012 Jan 15;188(2):753-64. doi: 10.4049/jimmunol.1101652. Epub 2011 Dec 14. J Immunol. 2012. PMID: 22174449
-
The hepatitis B virus X protein induces HIV-1 replication and transcription in synergy with T-cell activation signals: functional roles of NF-kappaB/NF-AT and SP1-binding sites in the HIV-1 long terminal repeat promoter.J Biol Chem. 2001 Sep 21;276(38):35435-43. doi: 10.1074/jbc.M103020200. Epub 2001 Jul 16. J Biol Chem. 2001. PMID: 11457829
-
[Effects of hepatitis B virus X protein on chronic hepatitis B pathophysiology].Med Clin (Barc). 2013 Jun 4;140(11):508-13. doi: 10.1016/j.medcli.2012.09.012. Epub 2012 Dec 12. Med Clin (Barc). 2013. PMID: 23245531 Review. Spanish.
-
Interference with nuclear factor kappaB signaling pathway by pathogen-encoded proteases: global and selective inhibition.Mol Microbiol. 2016 Feb;99(3):439-52. doi: 10.1111/mmi.13245. Epub 2015 Nov 5. Mol Microbiol. 2016. PMID: 26449378 Free PMC article. Review.
Cited by
-
Modulation of autophagy-like processes by tumor viruses.Cells. 2012 Jun 25;1(3):204-47. doi: 10.3390/cells1030204. Cells. 2012. PMID: 24710474 Free PMC article.
-
Phosphorylation and polyubiquitination of transforming growth factor beta-activated kinase 1 are necessary for activation of NF-kappaB by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor.J Virol. 2011 Mar;85(5):1980-93. doi: 10.1128/JVI.01911-10. Epub 2010 Dec 15. J Virol. 2011. PMID: 21159881 Free PMC article.
-
The Role of Plasminogen Activator Inhibitor 1 in Predicting Sepsis-Associated Liver Dysfunction: An Observational Study.Int J Environ Res Public Health. 2023 Mar 9;20(6):4846. doi: 10.3390/ijerph20064846. Int J Environ Res Public Health. 2023. PMID: 36981754 Free PMC article.
-
IL-23 enhances the malignant properties of hepatoma cells by attenuation of HNF4α.Oncotarget. 2018 Jun 19;9(47):28309-28321. doi: 10.18632/oncotarget.24875. eCollection 2018 Jun 19. Oncotarget. 2018. PMID: 29983862 Free PMC article.
-
The effect of CXCL9 on the invasion ability of hepatocellular carcinoma through up-regulation of PREX2.J Mol Histol. 2014 Dec;45(6):689-96. doi: 10.1007/s10735-014-9593-0. Epub 2014 Aug 24. J Mol Histol. 2014. PMID: 25151370
References
-
- Chisari F. V., Ferrari C. (1995) Annu. Rev. Immunol. 13, 29–60 - PubMed
-
- Tiollais P., Charnay P., Vyas G. N. (1981) Science 213, 406–411 - PubMed
-
- Luster A. D., Wang J., Zhao J. H., Wang P. P., Xiang G. J., Lee Y. H., Yun Y., Doria M., Klein N., Lucito R., Schneider R. J. (1998) N. Engl. J. Med. 338, 436–445 - PubMed
-
- Rossi D., Zlotnik A. (2000) Annu. Rev. Immunol. 18, 217–242 - PubMed
-
- Narumi S., Tominaga Y., Tamaru M., Shimai S., Okumura H., Nishioji K., Itoh Y., Okanoue T. (1997) J. Immunol. 158, 5536–5544 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

