A multifunctional mannosyltransferase family in Candida albicans determines cell wall mannan structure and host-fungus interactions
- PMID: 20164191
- PMCID: PMC2852947
- DOI: 10.1074/jbc.M109.081513
A multifunctional mannosyltransferase family in Candida albicans determines cell wall mannan structure and host-fungus interactions
Abstract
The cell wall proteins of fungi are modified by N- and O-linked mannosylation and phosphomannosylation, resulting in changes to the physical and immunological properties of the cell. Glycosylation of cell wall proteins involves the activities of families of endoplasmic reticulum and Golgi-located glycosyl transferases whose activities are difficult to infer through bioinformatics. The Candida albicans MNT1/KRE2 mannosyl transferase family is represented by five members. We showed previously that Mnt1 and Mnt2 are involved in O-linked mannosylation and are required for virulence. Here, the role of C. albicans MNT3, MNT4, and MNT5 was determined by generating single and multiple MnTDelta null mutants and by functional complementation experiments in Saccharomyces cerevisiae. CaMnt3, CaMnt4, and CaMnt5 did not participate in O-linked mannosylation, but CaMnt3 and CaMnt5 had redundant activities in phosphomannosylation and were responsible for attachment of approximately half of the phosphomannan attached to N-linked mannans. CaMnt4 and CaMnt5 participated in N-mannan branching. Deletion of CaMNT3, CaMNT4, and CaMNT5 affected the growth rate and virulence of C. albicans, affected the recognition of the yeast by human monocytes and cytokine stimulation, and led to increased cell wall chitin content and exposure of beta-glucan at the cell wall surface. Therefore, the MNT1/KRE2 gene family participates in three types of protein mannosylation in C. albicans, and these modifications play vital roles in fungal cell wall structure and cell surface recognition by the innate immune system.
Figures
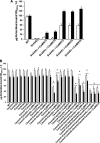


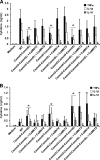
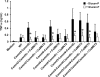
Similar articles
-
Biochemical characterization of recombinant Candida albicans mannosyltransferases Mnt1, Mnt2 and Mnt5 reveals new functions in O- and N-mannan biosynthesis.Biochem Biophys Res Commun. 2012 Mar 2;419(1):77-82. doi: 10.1016/j.bbrc.2012.01.131. Epub 2012 Feb 3. Biochem Biophys Res Commun. 2012. PMID: 22326920 Free PMC article.
-
The Mnn2 mannosyltransferase family modulates mannoprotein fibril length, immune recognition and virulence of Candida albicans.PLoS Pathog. 2013;9(4):e1003276. doi: 10.1371/journal.ppat.1003276. Epub 2013 Apr 25. PLoS Pathog. 2013. PMID: 23633946 Free PMC article.
-
Mnt1p and Mnt2p of Candida albicans are partially redundant alpha-1,2-mannosyltransferases that participate in O-linked mannosylation and are required for adhesion and virulence.J Biol Chem. 2005 Jan 14;280(2):1051-60. doi: 10.1074/jbc.M411413200. Epub 2004 Nov 1. J Biol Chem. 2005. PMID: 15519997 Free PMC article.
-
Mannosylation in Candida albicans: role in cell wall function and immune recognition.Mol Microbiol. 2013 Dec;90(6):1147-61. doi: 10.1111/mmi.12426. Epub 2013 Nov 8. Mol Microbiol. 2013. PMID: 24125554 Free PMC article. Review.
-
Fungal Mannosyltransferases as Fitness Attributes and their Contribution to Virulence.Curr Protein Pept Sci. 2017;18(11):1065-1073. doi: 10.2174/1389203717666160813164253. Curr Protein Pept Sci. 2017. PMID: 27526929 Review.
Cited by
-
Disruption of protein rhamnosylation affects the Sporothrix schenckii-host interaction.Cell Surf. 2021 Jun 26;7:100058. doi: 10.1016/j.tcsw.2021.100058. eCollection 2021 Dec. Cell Surf. 2021. PMID: 34308006 Free PMC article.
-
A Candida parapsilosis Overexpression Collection Reveals Genes Required for Pathogenesis.J Fungi (Basel). 2021 Jan 29;7(2):97. doi: 10.3390/jof7020097. J Fungi (Basel). 2021. PMID: 33572958 Free PMC article.
-
The Genomes of Three Uneven Siblings: Footprints of the Lifestyles of Three Trichoderma Species.Microbiol Mol Biol Rev. 2016 Feb 10;80(1):205-327. doi: 10.1128/MMBR.00040-15. Print 2016 Mar. Microbiol Mol Biol Rev. 2016. PMID: 26864432 Free PMC article. Review.
-
Unraveling unique structure and biosynthesis pathway of N-linked glycans in human fungal pathogen Cryptococcus neoformans by glycomics analysis.J Biol Chem. 2012 Jun 1;287(23):19501-15. doi: 10.1074/jbc.M112.354209. Epub 2012 Apr 12. J Biol Chem. 2012. PMID: 22500028 Free PMC article.
-
Inhibition of Dephosphorylation of Dolichyl Diphosphate Alters the Synthesis of Dolichol and Hinders Protein N-Glycosylation and Morphological Transitions in Candida albicans.Int J Mol Sci. 2019 Oct 12;20(20):5067. doi: 10.3390/ijms20205067. Int J Mol Sci. 2019. PMID: 31614738 Free PMC article.
References
-
- Pappas P. G., Rex J. H., Lee J., Hamill R. J., Larsen R. A., Powderly W., Kauffman C. A., Hyslop N., Mangino J. E., Chapman S., Horowitz H. W., Edwards J. E., Dismukes W. E. (2003) Clin. Infect. Dis. 37, 634–643 - PubMed
-
- Netea M. G., Brown G. D., Kullberg B. J., Gow N. A. (2008) Nat. Rev. Microbiol. 6, 67–78 - PubMed
-
- Klis F. M., de Groot P., Hellingwerf K. (2001) Med. Mycol. 39, (Suppl. 1) 1–8 - PubMed
-
- Calderone R. A. (1993) Trends Microbiol. 1, 55–58 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

