Dynamic regulation of CD45 lateral mobility by the spectrin-ankyrin cytoskeleton of T cells
- PMID: 20164196
- PMCID: PMC2857017
- DOI: 10.1074/jbc.M109.075648
Dynamic regulation of CD45 lateral mobility by the spectrin-ankyrin cytoskeleton of T cells
Abstract
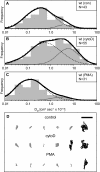
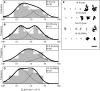
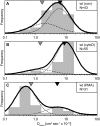
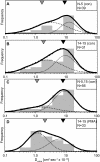
The leukocyte common antigen, CD45, is a critical immune regulator whose activity is modulated by cytoskeletal interactions. Components of the spectrin-ankyrin cytoskeleton have been implicated in the trafficking and signaling of CD45. We have examined the lateral mobility of CD45 in resting and activated T lymphocytes using single-particle tracking and found that the receptor has decreased mobility caused by increased cytoskeletal contacts in activated cells. Experiments with cells that have disrupted betaI spectrin interactions show decreased cytoskeletal contacts in resting cells and attenuation of receptor immobilization in activated cells. Applying two types of population analyses to single-particle tracking trajectories, we find good agreement between the diffusion coefficients obtained using either a mean squared displacement analysis or a hidden Markov model analysis. Hidden Markov model analysis also reveals the rate of association and dissociation of CD45-cytoskeleton contacts, demonstrating the importance of this analysis for measuring cytoskeleton binding events in live cells. Our findings are consistent with a model in which multiple cytoskeletal contacts, including those with spectrin and ankyrin, participate in the regulation of CD45 lateral mobility. These interactions are a major factor in CD45 immobilization in activated cells. Furthermore, cellular activation leads to CD45 immobilization by reduction of the CD45-cytoskeleton dissociation rate. Short peptides that mimic spectrin repeat domains alter the association rate of CD45 to the cytoskeleton and cause an apparent decrease in dissociation rates. We propose a model for CD45-cytoskeleton interactions and conclude that the spectrin-ankyrin-actin network is an essential determinant of immunoreceptor mobility.
Figures

Similar articles
-
The spectrin-ankyrin skeleton controls CD45 surface display and interleukin-2 production.Immunity. 2002 Sep;17(3):303-15. doi: 10.1016/s1074-7613(02)00396-5. Immunity. 2002. PMID: 12354383
-
Spectrin oligomerization is cooperatively coupled to membrane assembly: a linkage targeted by many hereditary hemolytic anemias?Exp Mol Pathol. 2001 Jun;70(3):215-30. doi: 10.1006/exmp.2001.2377. Exp Mol Pathol. 2001. PMID: 11418000
-
Tyrosine phosphatase activity of lymphoma CD45 (GP180) is regulated by a direct interaction with the cytoskeleton.J Biol Chem. 1992 Oct 25;267(30):21551-7. J Biol Chem. 1992. PMID: 1400466
-
Pleiotropic Ankyrins: Scaffolds for Ion Channels and Transporters.Channels (Austin). 2022 Dec;16(1):216-229. doi: 10.1080/19336950.2022.2120467. Channels (Austin). 2022. PMID: 36082411 Free PMC article. Review.
-
The molecular basis for membrane - cytoskeleton association in human erythrocytes.J Cell Biochem. 1982;18(1):49-65. doi: 10.1002/jcb.1982.240180106. J Cell Biochem. 1982. PMID: 6461664 Review.
Cited by
-
Coordinated roles for glycans in regulating the inhibitory function of CD22 on B cells.Biomed J. 2019 Aug;42(4):218-232. doi: 10.1016/j.bj.2019.07.010. Epub 2019 Sep 16. Biomed J. 2019. PMID: 31627864 Free PMC article. Review.
-
CD28 sensitizes TCR Ca²⁺ signaling during Ag-independent polarization of plasma membrane rafts.J Immunol. 2013 Sep 15;191(6):3073-81. doi: 10.4049/jimmunol.1300485. Epub 2013 Aug 21. J Immunol. 2013. PMID: 23966623 Free PMC article.
-
Analysis of molecular diffusion by first-passage time variance identifies the size of confinement zones.Biophys J. 2011 Mar 16;100(6):1463-72. doi: 10.1016/j.bpj.2011.01.064. Biophys J. 2011. PMID: 21402028 Free PMC article.
-
Mechanical modulation of receptor-ligand interactions at cell-cell interfaces.Biophys J. 2012 Mar 21;102(6):1265-73. doi: 10.1016/j.bpj.2012.02.006. Epub 2012 Mar 20. Biophys J. 2012. PMID: 22455909 Free PMC article.
-
Trajectory Analysis in Single-Particle Tracking: From Mean Squared Displacement to Machine Learning Approaches.Int J Mol Sci. 2024 Aug 8;25(16):8660. doi: 10.3390/ijms25168660. Int J Mol Sci. 2024. PMID: 39201346 Free PMC article. Review.
References
-
- Hermiston M. L., Xu Z., Weiss A. (2003) Annu. Rev. Immunol. 21, 107–137 - PubMed
-
- Lynes M. A., Tibbetts D. J., Swenson L. M., Sidman C. L. (1993) Cell Immunol. 151, 65–79 - PubMed
-
- Earl L. A., Baum L. G. (2008) Immunol. Cell Biol. 86, 608–615 - PubMed
-
- Irles C., Symons A., Michel F., Bakker T. R., van der Merwe P. A., Acuto O. (2003) Nat. Immunol. 4, 189–197 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

